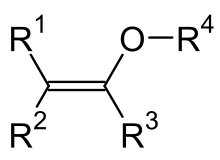
Enol ethers
As enol ethers are ethers of enols with the general structure R 1 R 2 C = CR 3 -O-R 4 , respectively. The vinyl ethers H 2 C = CH-O-R, in which a vinyl group ( ethenyl group ) is connected to a variable radical R via an oxygen atom, are of particular technical importance in this group of substances .
Extraction and presentation
Enol ethers can be prepared in the laboratory, for example, by reacting the carboxylic acid ester with Tebbe reagent .
Vinyl ethers are usually produced on an industrial scale by reacting the corresponding alcohols with ethyne in the presence of basic catalysts ( Reppe synthesis ).
The vinylation can be carried out either in the liquid or in the gas phase . For vinylation in the gas phase, basic heterogeneous catalysts such as potassium hydroxide on activated carbon or magnesium oxide / calcium oxide are used. In the liquid phase is the highly exothermic reaction is generally in the presence of alkali metal hydroxide , or - alkali metal alkoxide - catalysts performed.
Properties and use
Enol ethers and enamines are also known as electron-rich or activated alkenes because the oxygen atom adjacent to the double bond releases electrons to form a resonance structure (oxonium ion structure). This property gives the enol ethers a special reactivity, for example for the Diels-Alder reaction . An enol ether can be understood as the ether of the corresponding enolate. Enol ethers also serve as starting material for the preparation of α-bromocarboxylic acid esters, which in turn can be starting materials for the important Reformatzki reaction .
Allyl vinyl ethers are the starting substrates for Claisen rearrangements to γ, δ-unsaturated carbonyl compounds .
Vinyl ethers are used, among other things, as monomer building blocks in polymers and copolymers , in coatings, adhesives , printing inks and in radiation-curing paints . They are also used for the production of smells and flavors, pharmaceuticals and intermediate products in the chemical industry. The simplest representative of this group of substances, methyl vinyl ether , is often called simply “vinyl ether”.
literature
- MF Shostakovskii, Boris A. Trofimov, AS Atavin, VI Lavrov: Synthesis of Vinyl Ethers Containing Functional Groups and Heteroatoms . In: Russian Chemical Reviews . tape 37 , no. 11 , 1968, p. 907-919 , doi : 10.1070 / RC1968v037n11ABEH001713 .
Web links
Individual evidence
- ↑ Chapter: VINYL ETHERS-Production. In: Ullmanns Encyclopedia of Industrial Chemistry. 6. electronic edition.
- ^ Walter Reppe: Vinylierung . In: Justus Liebig's Annals of Chemistry . tape 601 , no. 1 , 1956, pp. 81-138 , doi : 10.1002 / jlac.19566010106 .
- ↑ Patent WO2004096741 : Continuous method for producing methyl vinyl ether. Released November 11, 2004 .