Cyclopentene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
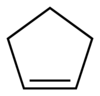
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclopentene | |||||||||||||||
| Molecular formula | C 5 H 8 | |||||||||||||||
| Brief description |
colorless liquid with a slightly sweet odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 68.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.77 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−135 ° C |
|||||||||||||||
| boiling point |
46 ° C |
|||||||||||||||
| Vapor pressure |
416 h Pa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (535 mg l −1 at 25 ° C) |
|||||||||||||||
| Dipole moment |
0.93 D (in carbon tetrachloride ) |
|||||||||||||||
| Refractive index |
1.4194 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cyclopentene is an organic compound with the empirical formula C 5 H 8 . It consists of a five-membered, unsaturated ring which has a double bond . In the homologous series of cycloalkenes , cyclopentene stands between cyclobutene and cyclohexene . Formally, it is a singly hydrogenated cyclopentadiene or a singly dehydrogenated cyclopentane . Cyclopentene has only a few uses.
presentation
Cyclopentene is by steam cracking of naphtha available, with about 2.2 kg per metric ton of naphtha cyclopentene incurred. On the other hand, it is produced more efficiently by the catalytic hydrogenation of cyclopentadiene.
properties
Cyclopentene enters into the usual reactions for cyclic alkenes, such as additions to the double bond and cycloadditions . Substitution reactions usually take place in the allylic position. By the catalytic oxidation of cyclopentene with oxygen can be cyclopentanone synthesize. The stereoselective formation of trans -Tricyclo [5.3.0.0 2,6 ] decane by photochemical dimerization of cyclopentene is a simple example of a photodimerization by [2 + 2] cycloaddition:
The reaction proceeds with a 55% yield.
Individual evidence
- ↑ a b c d e f g h Entry on cyclopentene in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d D. Hönicke, R. Födisch, P. Claus, M. Olson: Cyclopentadiene and Cyclopentene , in: Ullmanns Enzyklopädie der Technischen Chemie 2002 , Wiley-VCH, Weinheim.
- ↑ Data sheet Cyclopenten at AlfaAesar, accessed on March 2, 2010 ( PDF )(JavaScript required) .
- ↑ J. Smidt, W. Hafner, R. Jira, J. Sedlmeier, R. Sieber, R. Rüttinger, H. Kojer: Catalytic reactions of olefins on platinum metal compounds The Consortium process for the production of acetaldehyde , in: Angew. Chem. 1959 , 71 , 176-182; doi : 10.1002 / anie.19590710503 .
- ^ A b Albert Gossauer: Structure and Reactivity of Biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 143, ISBN 978-3-906390-29-1 .



