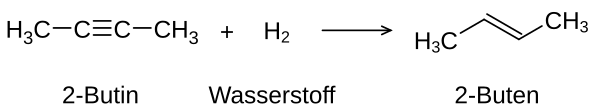Addition reaction
The chemical addition (from the Latin : addere = to add), also known as addition reaction or addition reaction , is one of the typical reactions in organic chemistry . In this reaction, at least two molecules are combined into one, with one or more multiple bonds being broken. The atom economy of the addition reactions is consistently excellent.
The addition is the reverse of the elimination , in which one molecule, e.g. B. with the formation of multiple bonds, molecules are split off.
In addition, for example , a symmetrical (for example HH, FF) or asymmetrical (for example H-Cl) molecule is attached to a covalent bond with a double or triple bond on an unsaturated hydrocarbon ( alkene and alkyne ), carboxylic acid derivative , aldehyde or ketone .
A special form of addition is polyaddition , in which several additions take place one after the other and lead to the formation of larger, chain-like molecules - up to macromolecules - which may also be spatially networked.
Example hydrocarbons
For the electron pairs not used by the carbon atoms , the alkenes and alkynes form double and triple bonds. You are now stable, but you do not have the maximum number of hydrogen atoms attached to each carbon atom. They are therefore called unsaturated.
Unsaturated hydrocarbons are stable, but energetically less favorable than saturated hydrocarbons. It is therefore easily possible to convert the multiple bonds of the unsaturated hydrocarbons to the next lower level by breaking a π bond (see double bond and covalent bond ), for example an alkene into an alkane.
Addition of hydrogen to an alkyne
The addition of hydrogen is also known as hydrogenation . A catalyst is usually used for the reaction ; the heterogeneous catalyst here is palladium (dispersed on activated carbon: Pd / C), which activates the hydrogen by binding it to the metal surface. In the case of heterogeneous catalysis, a cis -alkene is formed preferentially or exclusively .
Addition of hydrogen chloride to an alkene
Example aldehydes (acetal formation)
Aldehydes react - mostly acid-catalyzed - with alcohols to form hemiacetals , which can then react with another molecule of the corresponding alcohol to form the full acetals by splitting off water .
Applications
Proof of multiple bonds
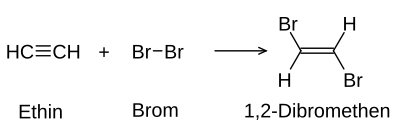
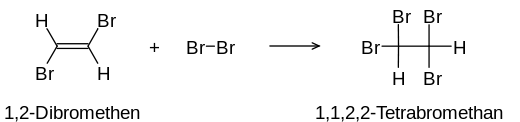
Multiple bonds in molecules of organic substances with bromine can be detected by means of an addition reaction : the normally reddish-brown bromine dissolved in water (" bromine water ") becomes discolored because the bromine splits the multiple bonds and accumulates and thus loses its color.
Both reactions continue until either all the bromine molecules are attached or there are no more multiple bonds (not desirable in the detection reaction). The positive result of the detection is ideally a colorless solution. Usually, however, the solution has a slightly yellowish color due to impurities that can hardly be avoided.
Types of addition reactions
Further reactions in organic chemistry
Other uses
See also
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd Edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 203, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 358, ISBN 3-342-00280-8 .