Glycerin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Glycerin | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 3 H 8 O 3 | ||||||||||||||||||
| Brief description |
colorless, sweet-tasting, slightly viscous liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 92.09 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.26 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
18 ° C |
||||||||||||||||||
| boiling point |
290 ° C (with decomposition) |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.4745 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Measured as the inhalable aerosol fraction:
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−669.6 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Glycerine (from the Greek. Glykeros "sweet", also glycerol or glycerine ) is the common name and the common name of propane-1,2,3-triol . Glycerin is a sugar alcohol and the simplest trihydric alcohol , a triol. The name glycerol was introduced because it has the correct ending -ol for an alcohol (the ending -in stands for alkynes or amines ).
Glycerine is found in all natural fats and oils - e.g. B. Vegetable oils - chemically bound as fatty acid esters ( triglycerides ) and play a central role as an intermediate in various metabolic processes . As a food additive , it has the abbreviation E 422 .
history
1779 was Carl Wilhelm Scheele in the saponification of olive oil for the first time glycerol. In 1813 Michel-Eugène Chevreul was able to prove that fats are esters of fatty acids and glycerine and gave the alcohol its name in 1823, derived from the Greek glykys 'sweet'. In 1836, Théophile-Jules Pelouze clarified the structure . The Englishman George Fergusson Wilson developed a process in 1854 to synthesize pure glycerine on an industrial scale. In the period that followed, interest in glycerine rose as a precursor to the then newly discovered nitroglycerine .
At that time, glycerine was mostly obtained from oils and fats, but the production volume was insufficient in times of war, so that for the first time plants were built in which glycerine was fermented from sugar. In 1943 a new, petroleum-based production method for glycerin was found in Germany, which subsequently replaced fermentative production. Around the turn of the millennium, around 25% of glycerine was made from petroleum.
Political measures to promote biodiesel also increased the amount of the by-product glycerine from natural sources. In the meantime, glycerine is produced almost exclusively from renewable raw materials. The glycerine price has fallen sharply due to the increased production volume. This makes glycerine interesting for new applications for which it used to be too expensive. In 2015 the global annual production of glycerine was around 4 million tons.
Extraction and presentation
The production can be done petrochemically from propene with the intermediate products allyl chloride and epichlorohydrin or chemically as a by- product in the saponification of natural fats and oils for the production of soaps (= alkali salts of fatty acids). In the past, animal fats were mainly used for this purpose.
Large quantities of glycerine are now produced as a by-product of biodiesel production. This is done by transesterifying mostly vegetable oils with methanol . A fat molecule ( triacylglyceride ) is converted with three methanol molecules to glycerine and three fatty acid methyl esters (FAME).

A biotechnological production by fermentation is possible. Yeasts can sulphite the fermentation of ethanol production switch to glycerol production. Molasses was often used as a substrate because it contains a lot of sulphite in addition to a high proportion of sugar. This form of fermentation in 1918 by Neuberg as a second fermentation Neuberg'sche form referred. The process goes back to Carl Neuberg , was developed by Karl Lüdecke and Wilhelm Connstein from the United Chemical Works in Berlin-Charlottenburg and was of great importance for the production of explosives in Germany during the First World War.
Glycerine is commercially available in various purities. For industrial purposes, it is as crude glycerol and pharmaceutical purposes ( pharmaceutical grade glycerine offered) in the qualities 99,8-, 99,5- and about 86-percent. 86 percent (with 14% water) it is technically easier to handle because of the greatly reduced melting point (−10 ° C) and the lower viscosity (approx. 100 mPas). The processing takes place through multi-stage distillation , deodorization and filtration . Highly pure, synthetic glycerine does not come from animal or vegetable precursors and is used particularly in quality-sensitive areas of the pharmaceutical industry as well as the cosmetics and food industries.
properties
Physical Properties
At room temperature, glycerine is a colorless, odorless, slightly viscous and hygroscopic liquid that tastes sweet. Glycerine has a viscosity of 1480 mPas (20 ° C).
Chemical properties
Glycerine forms white steam when exposed to heat. When heated under a lack of oxygen, it decomposes into the unsaturated aldehyde propenal , which is readily soluble in water (267 g / l) and is also toxic , which is also called acrylaldehyde or acrolein .
With solid potassium permanganate , it reacts completely to form carbon dioxide and water with spontaneous combustion.
It is only oxidized by aqueous potassium permanganate to mesoxalic acid. ( COD titration by manganometry )
Safety-related parameters
Glycerine forms flammable vapor-air mixtures at higher temperatures. The compound has a flash point of 191 ° C. The explosion range is between 2.6% by volume (99 g / m 3 ) as the lower explosion limit (LEL) and 11.3% by volume (435 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 400 ° C. The substance therefore falls into temperature class T2.
use
Food and cosmetics
Because of its water-binding properties, glycerine is contained in cosmetics as a moisturizer . As a food additive , glycerine is used under number E 422 to keep things moist, for example for dates or chewing gum, but also as a sweetener. Glycerine is also found in various toothpastes.
household
Often, glycerin is added to the water of Christmas tree stands to keep the tree fresher longer. The glycerine provides frost protection and makes the needles last longer. Due to its moisturizing effect, glycerine is used in leather care products and shoe creams to keep leather smooth and supple. A little glycerine is also usually added when making liquid for soap bubbles.
Tobacco, cigarettes and vaporizers
Glycerine (E 422) is used together with 1,2-propanediol as a humectant for tobacco products, not to be confused with glycerine-phosphoric acid and its sodium, potassium and magnesium compounds, the purity requirements of which are clearly defined in the Tobacco Ordinance . In cigarette and pipe tobacco, the main purpose of the humectants is to extend the storage times of the products and prevent them from drying out. Shisha tobacco is mixed with significantly higher quantities of humectants by the manufacturers, on the one hand to prevent the tobacco from burning and on the other hand to generate a denser vapor. Like 1,2-propanediol, glycerine is also used as a smoke fluid in electronic cigarettes .
Industry and technology
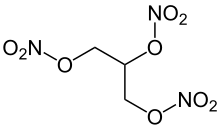
Glycerin is used as an antifreeze (mixed with water as a heat transfer medium), lubricant and plasticizer . In fog fluids it is added to increase the durability of the fog. The substance is required as a reactant in the manufacture of plastics , microchips , dyes and toothpaste . The reaction with a mixture of concentrated nitric acid and concentrated sulfuric acid produces "glycerol trinitrate". This compound is the explosive known as " nitroglycerin " , which together with diatomaceous earth forms the explosive dynamite .
Due to the significantly lower prices at times, new areas of application for glycerine are being sought. In addition to incineration, use as an additional nutrient medium (cosubstrate) in biogas plants for the production of biogas and use as a fermentation substrate in industrial biotechnology are alternatives.
Research is also being carried out into converting glycerine with isobutene to glycerine tert-butyl ether (GTBE; analogous to MTBE and ETBE ) for use as a fuel additive. Since 2009, Volkswagen AG has been using glycerine obtained from old frying fats instead of ethylene glycol obtained from petroleum as a coolant additive (G13) in its vehicle models.
Glycerine can be converted into a basic chemical building block - allyl alcohol - in a new process under the action of formic acid .
Agriculture
With increasing feed prices, glycerine is attracting interest as feed for ruminants, pigs and chickens.
medicine
Glycerine is used in medicine as a medicinal substance for the treatment of cerebral edema . To do this, it is infused as a 10% solution .
In the form of glycerol-suppository it comes as a laxative ( laxative ) are used. The effect arises, first, by a reflex effect: Due to the contact of glycerol with the rectal mucosa, the Defäkations lovely increased (see also: defecation reflex ). On the other hand, there is an osmotic effect: the water flowing into the intestinal lumen makes the stool softer and smoother.
The subject of medical research is the use of glycerine to maintain human brain and organ functions during an artificial lowering of body temperature. This could be important for lengthy, difficult medical operations. The biological model is the Canadian gray tree frog Hyla versicolor , whose body cells are enriched with glycerine for the winter.
Glycerine is also used as part of the Klockhoff test (glycerol stress test) to diagnose Menière's disease .
Platform chemical in the chemical industry
Glycerine is a platform chemical because it is obtained on a large scale using bio-based methods and is chemically suitable as a synthetic building block for numerous different end products.
Biological importance
Most animal and vegetable fats and oils are triacylglycerides (triglycerides). They consist of the trihydric alcohol glycerine, which is threefold esterified with fatty acids via the hydroxyl groups (-OH) . They are energy stores in adipose tissue or in the seeds of oil plants such as rapeseed, soy, sunflower, coconut palms or oil palms. Phosphoglycerides have a similar structure . Instead of the third fatty acid, a phosphate group is esterified and a residue is coupled to it, like choline in lecithin . This gives the molecule a polar and an apolar region, which enables the formation of a membrane ( cell membrane ).
Almost all naturally occurring glycerol derivatives have the sn configuration , which defines the spatial arrangement of the substituents on the central carbon atom of the glycerol.
Some insects contain glycerine in their blood as a natural antifreeze to help them survive low temperatures. Hornets survive down to −17 ° C and arctic ground beetles down to −85 ° C.
Health risks
In food
As an additive in food, glycerine is considered harmless. It is classified as harmless by the Food and Drug Administration (US Food Control Authority ) with no maximum quantity restriction ( permitted daily dose ). In the EU, it is also approved as an additive E422 without a maximum amount restriction; this was confirmed again in a study in 2017.
The physiological calorific value of glycerine is 17 kJ or 4 kcal per gram.
In hookah tobacco
Glycerine, along with other humectants, is added to water pipe tobacco in considerable quantities. A study by the Federal Institute for Risk Assessment (BfR) quantified the humectant content in water pipe smoke and was able to detect significant amounts of the humectants glycerine and propanediol (propylene glycol) . The evaluation of the results pointed to possible health risks, for example changes in the cell epithelium in the larynx and irritation of the nasal mucous membranes up to nosebleeds were found in animal experiments .
Web links
Individual evidence
- ↑ Entry on E 422: Glycerol in the European database for food additives, accessed on August 6, 2020.
- ↑ Entry on GLYCERIN in the CosIng database of the EU Commission, accessed on February 17, 2020.
- ↑ a b c d e f g Entry on glycerol. In: Römpp Online . Georg Thieme Verlag, accessed on February 10, 2013.
- ↑ a b c d e f g h i j k Entry for CAS no. 56-81-5 in the GESTIS substance database of the IFA , accessed on March 20, 2019(JavaScript required) .
- ^ Organikum - organic-chemical basic internship, 16th edition, Deutscher Verlag der Wissenschaften Berlin 1986, p. 648.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 9, 2015.
- ^ Entry on glycerine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Margarida Bastos, Sven-Ove Nilsson, Maria DMC Ribeiro da Silva, Manuel AV Ribeiro da Silva, Ingemar Wadsö: Thermodynamic properties of glycerol enthalpies of combustion and vaporization and the heat capacity at 298.15 K. Enthalpies of solution in water at 288.15, 298.15 , and 308.15 K . In: The Journal of Chemical Thermodynamics . tape 20 , no. 11 , 1988, pp. 1353-1359 , doi : 10.1016 / 0021-9614 (88) 90173-5 .
- ↑ duden.de: Glycerine
- ^ A b c d e Mario Pagliaro: Glycerol - The Renewable Platform Chemical . Elsevier, 2017, ISBN 978-0-12-812205-1 .
- ^ Anita McConnell: Wilson, George Fergusson (1822-1902). In: Oxford Dictionary of National Biography . Oxford University Press, 2004; online edn, May 2009 accessed 19 April 2015 .
- ↑ a b Arno Behr & Thomas Seidensticker: Introduction to the chemistry of renewable raw materials - occurrence, conversion, use . Springer Spectrum, 2018, ISBN 978-3-662-55254-4 , 5. The by-product of oleochemistry - glycerine, p. 85-105 .
- ^ Hans G. Schlegel: General Microbiology , editor: Georg Fuchs, Georg Thieme Verlag , Stuttgart; 8. Fully redesigned & extended edition published in September 2006, ISBN 3-13-444608-1 .
- ↑ a b c What is glycerin. ( Memento of February 2, 2010 in the Internet Archive ) Deutsche Molasse Handelsgesellschaft (DMH), accessed on January 12, 2010.
- ↑ The OPTIM Advantage. ( Memento of May 25, 2011 in the Internet Archive ) The Dow Chemical Company, accessed October 12, 2010.
- ↑ Chemsafe database for safety-related parameters in explosion protection, PTB Braunschweig / BAM Berlin, accessed on March 20, 2019.
- ↑ a b E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Development of GTBE from glycerine; Report in the innovation report .
- ↑ VW employees improve the CO 2 balance of the vehicle range with an innovative coolant additive .
- ↑ New method allows the production of chemically interesting basic building blocks from glycerine and erythritol .
- ↑ Gregory Cevc: Phospholipids Handbook. CRC Press, 1993, ISBN 978-0-8247-9050-9 , p. 2.
- ↑ Sec. 182.1320 glycerin. FDA, accessed March 4, 2018 .
- ↑ EFSA Panel on Food Additives and Nutrient Sources added to Food: Re-evaluation of glycerol (E 422) as a food additive. In: EFSA Journal. March 15, 2017, accessed March 4, 2018 .
- ↑ BfR press release of August 3, 2011 .