Propionic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
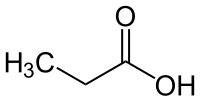
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Propionic acid | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 3 H 6 O 2 | |||||||||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 74.08 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.99 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
−21 ° C |
|||||||||||||||||||||
| boiling point |
141 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| pK s value |
4.87 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.386 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−510.7 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Propionic acid is the common name of propanoic acid , a carboxylic acid with a pungent odor. Their salts and esters are called propionates or systematically propanoates .
History and etymology
Johann Gottlieb discovered propionic acid and its salts in 1844 through the reaction of carbohydrates with molten alkali metal hydroxides . The name propionic acid was given to it in 1847 by the French chemist Jean-Baptiste Dumas . Dumas derived it from the Greek protos , 'the first', and pion , 'fat', since it is the smallest (first) carboxylic acid, which behaves similarly to fatty acids, in that when it is salted out, it forms an oil film on water and a soap-like one Forms potassium salt.
Occurrence
Propionic acid occurs naturally in some essential oils . There are also bacteria that produce propionic acid, such as clostridia , which colonize the human large intestine . There they form the acid from undigested carbohydrates . The formation of propionic acid by certain bacteria is also important in the production of certain cheeses : Propionic acid bacteria in the cheese curd form the characteristic holes and aroma of Emmentaler and other hard cheeses by releasing carbon dioxide and propionic acid. It also forms during fermentation and fermentation processes or during the biological breakdown of plant or animal materials.
In addition to butyric acid , hydrogen sulfide and other volatile organic compounds containing sulfur ( methanethiol , dimethyl sulfide ), one cause of the unpleasant halitosis in humans is propionic acid.
Extraction and presentation
Industrial manufacture
Two processes are currently used for the large-scale production of propionic acid in the chemical industry . The hydrocarboxylation of ethene (carbonylation in the presence of water) was developed in the 1930s by Walter Reppe at BASF in Ludwigshafen am Rhein . Due to the inexpensive availability of aldehydes based on petrochemical raw materials through the development of oxo synthesis or hydroformylation , propionic acid is now also produced by the oxidation of propionaldehyde .
Hydrocarboxylation of ethene (BASF process)
As part of this process, ethene is reacted with carbon monoxide and water at temperatures of 250-320 ° C and pressures of 100-300 bar in the presence of nickel tetracarbonyl [Ni (CO) 4 ] as a homogeneous catalyst in the liquid phase.
Today, this process is mainly carried out in plants belonging to BASF SE . The company is the world's largest manufacturer of propionic acid and produces it at its Verbund sites in Ludwigshafen am Rhein ( Germany ) and Nanjing ( China ). The production capacity of the BASF plants is estimated at around 150,000 tons per year.
Oxidation of propionaldehyde
Another important process for the large-scale production of propionic acid is the oxidation of propionaldehyde . The latter is currently produced from petrochemical raw materials and is therefore inexpensive and available in large quantities. The liquid phase oxidation of propionaldehyde is carried out with atmospheric oxygen at mild temperatures of 30-50 ° C and low pressures of 1-3 bar in the presence of manganese (II) propionate as a catalyst.
You work in the liquid phase and use propionic acid as a solvent .
Others
Propionic acid is also formed when biogas is obtained from organic waste. Propionic acid is produced in the second phase of material decomposition, the acid-forming phase, in an airtight fermentation tank.
properties
Safety-related parameters
Propionic acid forms flammable vapor-air mixtures at high temperatures. The compound has a flash point of 52 ° C. The explosion range is between 2.85% by volume (87 g / m 3 ) as the lower explosion limit (LEL) and 12% by volume (370 g / m 3 ) as the upper explosion limit (UEL). This results in an upper explosion limit Explosion point of 48 ° C. The limit gap width was determined to be 1.1 mm. This results in an assignment to explosion group IIA. The ignition temperature is 485 ° C. The substance therefore falls into temperature class T1.
use
Propionic acid is an important building block in the synthesis of plastics , herbicides and pharmaceuticals . Propionic acid (E 280) and its salts sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) are used as preservatives . The acid itself has an unpleasant taste for humans, which is why the salts of the acid are used in the food industry, especially for packaged sliced bread and pastries. The acid itself is often added to the silage , where the proportion can be up to 2% of the dry matter. The additive has the positive side effect that it prevents ketoacidosis in dairy cattle. In the Federal Republic of Germany, propionic acid and its salts were banned in sliced bread from 1988, as it was reported that they cause cancer-like changes in the forestomach in rats. According to the latest EU law, it is allowed again. Propionic acid is also classified as safe by the American Food and Drug Administration . Humans need vitamin B12 to break down propionic acid . In addition, propionic acid and its salts are approved as preservatives for cosmetics according to the German Cosmetics Ordinance .
Many mushrooms are able to grow on pure propionic acid. However, especially in connection with other carbon sources such as glucose , the polyketide synthase of the fungi and thus growth is inhibited.
The esters of propionic acid are used as odoriferous substances , aromatic substances and as solvents .
Human medicine
Investigations on cell lines of small and large intestine cells have shown that the stimulation of the G protein- coupled receptors for short-chain fatty acids GRP41 (free fatty acid receptor FFAR 3) and GRP43 (free fatty acid receptor FFAR 2) by propionic acid, for example, is beneficial Influencing the fat and sugar metabolism leads. The same observation could also be made directly on rats.
The increased formation of two hormones, PYY ( peptide YY ) and GLP-1 ( glucagon-like peptide 1 ) is particularly important in this context. PYY and GLP-1 are produced in the “L cells” of the intestine, especially in the last part of the small intestine (ileum) and in the large intestine (appendix, ascending colon). GLP-1 activates insulin production in the pancreas and at the same time inhibits glucagon formation there (glucagon is the insulin counterpart and increases the blood sugar level). Thus, the blood sugar level is lowered by short-chain fatty acids .
At the same time, the appetite is reduced and the feeling of satiety is increased. PYY and GLP-1 work both in the hypothalamus , a certain brain region, in the sense of a feeling of satiety and a reduction in appetite, and in the stomach, where emptying is inhibited.
If propionic acid is added to the diet in the form of sodium propionate or calcium propionate , this results in the production of PYY and GLP-1, the levels of which rise in the blood. Over the course of six months, obese people lose weight, including in the abdomen and liver, and the insulin resistance, which worsens in the control group, remains the same.
Tirosh et al. showed in a study with 14 volunteers that the consumption of a mixed meal containing 1000 mg propionate shortly after eating in humans led to an increase in plasma glucagon , a gluconeogenic hormone called fatty acid binding protein 4 (FABP4), and to the release of noradrenaline by the sympathetic Nervous system led. This in turn caused insulin resistance with compensatory hyperinsulinemia . The results suggest that propionate may act as a metabolic disruptor, potentially increasing the risk of type 2 diabetes mellitus and obesity in humans.
Anti-inflammatory effect
Propionic acid is formed by bacteria in the large intestine in a diet rich in fiber and is then one of the most important sources of energy for the superficial intestinal cells (intestinal epithelia). Like other short-chain fatty acids, propionic acid also has a regulating influence on the inflammation of the intestine and the entire organism and can Animal experiments prevent chronic inflammatory diseases such as multiple sclerosis . Recently, there have even been successes in the therapy of human MS. In addition, propionic acid stimulates certain neuroendocrine cells of the large intestine, the so-called L-cells, to produce hormones (glucagon-like peptides 1, peptides YY), which are beneficial for obesity and diabetes impact.
Cardiovascular diseases
In an animal experiment, researchers fed propionate to mice with high blood pressure . After that, the animals had less pronounced heart damage or abnormal enlargement of the organ, which made them less prone to cardiac arrhythmias . Vascular damage, such as B. atherosclerosis decreased in mice. The research team now hopes to confirm their results by studying the effects of the substance on humans.
Physiological effects
If people eat a high-fiber diet, after a few months the composition of the bacteria in the intestine changes and more short-chain fatty acids are formed.
The epithelial cells of the large intestine absorb almost 90% of the short-chain fatty acids and pass them on to the organism via the portal vein system and the liver. According to current estimates, humans get up to ten percent of their daily energy needs from short-chain fatty acids. In addition, the epithelia of the large intestine cover more than half of their energy requirements from short-chain fatty acids, in particular from butyric acid .
A number of cells have receptors on their surface that allow them to recognize short-chain fatty acids. These receptors transmit signals inside the cell that change the cell's behavior. It is interesting that these receptors are present on the one hand on cells that are involved in the metabolism of fat and sugar, and on the other hand they are also found on immune cells, for example: They are primarily so-called G-protein-coupled receptors (GPR) , especially GPR41 (free fatty acid receptor FFAR 3) and GPR43 (free fatty acid receptor FFAR 2).
GPR41 is found in cells of adipose tissue, pancreas, spleen , lymph nodes , bone marrow, lymphocytes and monocytes . GPR43 is found in the distal ileum , colon , adipose tissue, in monocytes and neutrophils (highest expression). Accordingly, the effects of propionic acid and its salts, such as sodium propionate , and other short-chain fatty acids, especially on the sugar and lipid metabolism and the immune system, have moved to the center of current research.
safety instructions
Propionic acid has a corrosive effect and, when diluted, irritates skin, eyes, mucous membranes and respiratory tract. With prolonged administration of propionic acid and propionates in the feed of rats in doses between 0.6 and 5%, these cause changes in the forestomach ( thickening and inflammation). However, this is classified as a species-specific reaction for rats, since no such effects were observed in other animal species such as mice and rabbits.
See also
Individual evidence
- ↑ Entry on E 280: Propionic acid in the European database for food additives, accessed on June 27, 2020.
- ↑ entry to propionic acid in the CosIng database of the European Commission, accessed on 22 April 2020th
- ↑ a b c d e f g h i j k l m n o p q r s t Entry on propionic acid in the GESTIS substance database of the IFA , accessed on October 7, 2018(JavaScript required) .
- ↑ U.-R. Samel, W. Kohler, AO Gamer, U. Keuser: Propionic Acid and Derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2012, doi : 10.1002 / 14356007.a22_223.pub2 .
- ↑ a b c Entry on propionic acid. In: Römpp Online . Georg Thieme Verlag, accessed on May 12, 2014.
- ↑ Entry on Propionic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 79-09-4 or propionic acid ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ^ Development of Systematic Names for the Simple Alkanes. ( Memento of the original from March 16, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag, 2011, ISBN 978-3-8348-1245-2 , pp. 61-62.
- ↑ Ulf ‐ Rainer Samel, Walter Kohler, Armin Otto Gamer, Ullrich Keuser, Shang ‐ Tian Yang, Ying Jin, Meng Lin Zhongqiang Wang, Joaquim Henrique Teles: Propionic Acid and Derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley ‐ VCH Verlag GmbH & Co. KGaA., January 31, 2018, doi : 10.1002 / 14356007.a22_223.pub4 .
- ^ Propionic acid pure. In: BASF product search. 2014, accessed March 21, 2020 .
- ↑ BASF expands capacity for propionic acid. CHEMIE TECHNIK, November 28, 2007, accessed on March 21, 2020 .
- ↑ a b Manfred Fedtke, Wilhelm Pritzkow, Gerhard Zimmermann: Technical organic chemistry - basic materials, intermediate products, final products, polymers . 1st edition. German publishing house for basic industry, Leipzig 1992, p. 133, ISBN 3-342-00420-7
- ↑ Stephan Ahlert, Rita Zimmermann, Johannes Ebling, Helmut König: Analysis of propionate-degrading consortia from agricultural biogas plants. In: MicrobiologyOpen. 5 (6), 2016, doi: 10.1002 / mbo3.386 .
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ EFSA: Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives. EFSA, December 2014, accessed December 2014 .
- ↑ Food and Drug Administration FDA: DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE. In: CFR - Code of Federal Regulations. Food and Drug Administration FDA, April 1, 2015, accessed April 1, 2015 .
- ↑ A. Psichas, ML Sleeth, KG Murphy a. a .: The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. International Journal of Obesity . In: International Journal of Obesity (Ed.): International Journal of Obesity . No. 39 . MacMillan Publishers, London, UK 2015, pp. 424-429 , doi : 10.1038 / ijo.2014.153 .
- ↑ Edward S. Chambers, Alexander Viardot et al. a .: Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults . In: Good . tape 64 , no. 11 , 2015, p. 1744–1754 , doi : 10.1136 / gutjnl-2014-307913 (English).
- ↑ Amir Tirosh1 †, Ediz S. Calay et al .: The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. In: Science Translational Medicine. April 24, 2019, accessed April 25, 2019 .
- ↑ Cell , Vol. 180, issue 6, pp. 1067-1080 (2020), DOI: https://doi.org/10.1016/j.cell.2020.02.035 .
- ↑ EN Bergman: Energy contributions of volatile fatty acids from the gastrointestinal tract in various species . In: Physiological Reviews . tape 70 , 1990, pp. 567-590 , PMID 2181501 .
- ↑ Hendrik Bartolomaeus et al .: The Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. In: Circulation. 2018, doi: 10.1016 / j.cardiores.2006.06.030 .
- ↑ Maria De Angelis, Eustacchio Montemurno, Lucia Vannini a. a .: Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome . In: Applied and Environmental Microbiology . tape 81 , no. 22 , 2015, p. 7945-7956 , doi : 10.1128 / AEM.02507-15 , PMID 26386056 .
- ↑ JG LeBlanc, F. Chain, R. Martín, LG Bermúdez-Humarán, S. Courau, P. Langella: Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. In: Microbial cell factories. Volume 16, number 1, May 2017, p. 79, doi: 10.1186 / s12934-017-0691-z , PMID 28482838 , PMC 5423028 (free full text) (review).
- ^ E. Patterson, JF Cryan, GF Fitzgerald, RP Ross, TG Dinan, C. Stanton: Gut microbiota, the pharmabiotics they produce and host health. In: The Proceedings of the Nutrition Society. Volume 73, number 4, November 2014, pp. 477-489, doi: 10.1017 / S0029665114001426 , PMID 25196939 (review), PDF .
- ↑ Olga Brandstätter, Oliver Schanz, Julia Vorac u. a .: Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor . In: Scientific Reports . tape 6 , 2016, p. 26091 , doi : 10.1038 / srep26091 .
- ^ R. Corrêa-Oliveira, JL Fachi, A. Vieira a. a .: Regulation of immune cell function by short-chain fatty acids. In: Clinical & translational immunology. Volume 5, number 4, April 2016, p. E73, doi: 10.1038 / cti.2016.17 , PMID 27195116 , PMC 4855267 (free full text) (review).
- ↑ H.-G. Classen, PS Elias, WP Hammes, M. Winter: Toxicological-hygienic assessment of food ingredients and additives. Behr's Verlag, 2001, ISBN 978-3-86022-806-7 .




