Neutrophil granulocyte
Neutrophil granulocytes , also called neutrophils for short , are specialized immune cells in vertebrates. In humans, they are the most common white blood cells ( leukocytes ), with a proportion of 50–65% . They are part of the innate immune system and are used to identify and destroy microorganisms . The name refers to the good stainability with neutral dyes ("neutral loving") and is a differentiation to eosinophils and basophils granulocytes .
Three mechanisms are known by which they can fight microorganisms. As phagocytes ("scavenger cells") they can ingest and digest them. In addition, the eponymous granule vesicles , which are stored in the cytoplasm , contain various substances that can kill microorganisms. When these substances are released into the environment, microorganisms are also damaged. Finally, in a process known as NETosis, neutrophils can form so-called "neutrophil extracellular traps" (English for neutrophil extracellular traps). These chromatin structures can bind some microorganisms and thereby render them harmless.
structure
Neutrophils are spherical cells 9 to 15 µm ( micrometers ) in diameter . The nucleus, which consists of three to five segments, is characteristic of mature neutrophils. The cytoplasm of neutrophils includes two types of granules : The "specific" granules, which are also referred to as secondary granules contain enzymes such as lysozyme , collagenase , lactoferrin , elastase , plasminogen activators , neuraminidase and cathepsin G . These granules cannot be stained with basic and acidic dyes, which is what differentiates them from basophils and eosinophils in their eponymous way . The "azurophilic" granules correspond to the lysosomes and are also called primary or non-specific granules. In addition to components such as acid hydrolases and antimicrobial enzymes, they also contain substances such as defensins , myeloperoxidase , and cathelicidins , which enable them to act effectively against some bacteria , viruses and fungi .
In rabbits , guinea pigs , chinchillas and ferrets , the neutrophil granules are acidophilic and stained with the dye eosin , so that we also speak of pseudo- eosinophils or heterophils .
maturation
An adult human produces more than 10 11 (one hundred billion) neutrophils per day. They are made in the bone marrow . Young neutrophils have a rod-shaped nucleus, which is why they are referred to as rod-nucleus; analogously, mature neutrophils with their three to five core segments are referred to as segment nuclei. If neutrophils do not come into contact with infections and / or inflammatory reactions within 6 to 8 hours of entering the bloodstream , they leave the bloodstream, experience programmed cell death ( apoptosis ) and are broken down by macrophages in the liver or spleen. Neutrophils generally have a lifespan of one to four days.
function
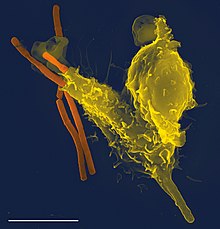
Neutrophils circulate in the blood and, in the event of an infection, migrate from the bloodstream into the tissue at the location where it occurred. This also applies to monocytes . There they pick up the microbes that cause the infection and digest them. In order to reach infected and / or inflamed tissue, both leave the bloodstream and its vessels in a multi-stage process mediated by adhesion molecules and chemokines (soluble "attractants"). They migrate through the intercellular spaces of the endothelial cells in postcapillary venules . The process of recruiting neutrophils and monocytes to the focus of infection can be divided into four steps:
- Selectin -mediated “Along” rolling on the vascular endothelium: Macrophages that have digested microbes on site release cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF). These induce an increase in selectins (P-selectin and E-selectin) on the surface of nearby endothelial cells. Neutrophils and monocytes have L-selectin on their surface as an adhesion molecule and the carbohydrate ligands specific for P- and E-selectin. The resulting selectin-selectin interactions are very weak and are interrupted by the shear forces of the bloodstream. This leads to a slower "along" rolling of neutrophils and monocytes on the endothelium, in that they permanently bind to the surface and then loosen again.
- Chemokine mediated enhancement of integrin - affinity : Distributed cytokines such as IL-1 and TNF induce macrophages, endothelial and other cell types, the production of chemokines. They are bound to the surface of the epithelial lumen and thereby concentrated. Neutrophils and macrophages rolling "past" recognize them with specific chemokine receptors. This leads to the fact that integrins on their surface change from a low affinity to a high affinity. In addition, these integrins accumulate and lead to stronger binding to the endothelial surface and slower “rolling”.
- Stable integrin-mediated adhesion to the endothelium: Parallel to the integrin affinity modification on neutrophils and monocytes (on their cell surface ligands such as VCAM-1 Engl. Vascular cell adhesion molecule-1) and ICAM-1 ( engl. Intercellular adhesion molecule-1) that bind to integrins of the endothelium. This ligand production is also induced by chemokines. Together with the effects described in 2, this leads to a firm bond to the endothelium. This results in rearrangements of the cytoskeleton and the leukocytes position themselves flattened on the endothelium.
- Migration of the endothelial tissue: Neutrophils and monocytes now follow the concentration gradients of the local chemokines and migrate between the endothelial cells to the infected tissue. For this process, parts of the extracellular matrix ( ECM ) of the endothelial cells are dissolved in order to provide enough space for the wandering leukocytes. For this purpose, they release their specific granules, the proteases of which enable the dissolution.
Through these processes, neutrophils and monocytes accumulate at the site of infection, which is the main component of inflammation . Due to the temporal differences in the induced chemokine receptor and integrin expression, neutrophils are first recruited to the site of infection within hours to days, and monocytes within days to weeks. In the event of an infection, the concentration of neutrophils in the blood rises ( neutrophilia ), which is demonstrated by an increase in "rod nuclei" (a characteristic of young neutrophils) that there is an increased formation of new cells. Once at the site of infection, microbes are ingested and destroyed by neutrophils and macrophages, a process called phagocytosis .
Neutrophil granulocytes also have the ability to bind bacteria by means of a released fibrillar matrix of granule proteins and chromatin . On the one hand, this prevents the further spread of bacteria and, on the other hand, promotes the destruction of the bacteria stuck there. These networks are English. "Neutrophil Extracellular Traps" (NETs). Based on the two other types of cell death, necrosis and apoptosis, this process is increasingly referred to in German literature as netosis and thus represents one of the three forms of cell death. Bacteria that produce DNAse can evade the defense mechanism by cutting the fibrillary matrix.
NETs - Neutrophil Extracellular Traps
Neutrophil Extracellular Traps, abbreviated to NETs (in German neutrophil extracellular traps , where the abbreviation NET is also reminiscent of net) are networks of extracellular fibers that bind pathogens and consist mainly of the DNA of neutrophil granulocytes.
It has long been known that neutrophils, who are at the forefront of fighting infection, have two different strategies to fight invading pathogens: phagocytosis of the microbes or secretion of antimicrobial substances into the environment. In 2004, the creation of NETs was described as a new mechanism. Neutrophils kill extracellular pathogens and only minimally damage the body's own cells. When activated in vitro with phorbol myristate acetate (PMA), interleukin -8 (IL-8) or lipopolysaccharide (LPS), neutrophils release granular proteins and chromatin to form an extracellular, fibrous matrix through an active process that NETs. NETs neutralize pathogens by using antimicrobial proteins bound to nuclear DNA, such as neutrophil elastase and histones . Immunofluorescence analyzes confirmed that NET proteins contain azurophil granules such as neutrophil elastase, cathepsin G and myeloperoxidase . In addition, proteins of specific granules such as lactoferrin , tertiary granules such as gelatinase , but no CD63 , actin , tubulin and also no other cytoplasmic proteins are contained. NETs ensure a high, local concentration of antimicrobial substances. They bind, immobilize and kill microbes extracellularly. The process is independent of the uptake by phagocytosis. In addition to their antimicrobial properties, NETs appear to provide a physical barrier that prevents further spread of the pathogen. In addition, the immobilization of granular proteins by the NETs prevents potentially harmful proteins, such as proteases , from spreading. This reduces the damage to the tissues adjacent to the area of inflammation . High-resolution electron microscopy images showed that NETs consist of strands of DNA and globular protein domains with a diameter of 15–17 nm and 25 nm. These combine to form larger structures with a diameter of 50 nm. However, NETs can also form much larger structures in the bloodstream that can be several hundred nanometers long and wide. Since NETs in the blood vessels could disrupt the blood flow, the NETosis in plasma and serum is much weaker.
It has been shown that not only bacteria but also pathogenic fungi such as Candida albicans cause neutrophils to develop NETosis, trapping and killing both hyphal and yeast-shaped cells. The formation of NETs has also been documented in connection with Plasmodium falciparum infections in children.
NETs could also have a harmful effect on the organism because exposure to extracellular histone complexes could contribute to the development of autoimmune diseases such as lupus erythematosus . NETs can also be implicated in inflammatory diseases. For example, NETs were found in patients with preeclampsia , an inflammatory disease during pregnancy that was known to have activated neutrophils. The involvement of NETs in the induction of antinuclear antibodies has also been demonstrated in children with malaria .
While it was originally assumed that NETs are formed in tissue in connection with bacterial or fungal infection, it has been shown that NETs can also form in the blood vessels, especially the lung capillaries and liver sinusoids , during sepsis . Intravascular NET formation is tightly controlled and regulated by platelets, which detect dangerous infections using TLR4, then bind to neutrophils and activate NET formation. Platelet-induced NETosis is very rapid (within minutes) and the neutrophils survive. NETs formed in blood vessels can immobilize bacteria in the bloodstream. The trapping of the bacteria could be demonstrated directly in flow chambers in vitro, and intravital microscopy revealed that the bacteria are found in the liver sinusoids and lung capillaries (sites where platelets bind neutrophils).
These observations suggest that NETs play an important role in the pathogenesis of infectious, inflammatory and thrombotic diseases.
Primary diseases
The septic granulomatosis leads despite a functioning hike to the site of infection and the inclusion of microbes in neutrophils to a defect in the breakdown, the "digestion". Neutrophils are therefore no longer available as part of the immune response, which means that infections can take a critical course.
See also
literature
- Abul K. Abbas, Andrew H. Lichtman, Shiv Pillai: Cellular and Molecular Immunology. 6th edition. Saunders Elsevier , Philadelphia 2007. ISBN 0-7216-0008-5
- S. Massberg et al .: Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases . In: Nature PG / Nature Medicine , 16, August 1, 2010, pp. 887-896, doi: 10.1038 / nm.2184 . "Also implicated in thrombotic processes". (English)
Web links
- Video: Neutrophil Granulocytes - Homo sapiens . Institute for Scientific Film (IWF) 1961, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / E-402 .
Individual evidence
- ↑ B. Amulic, C. Cazalet, GL Hayes, KD Metzler, A. Zychlinsky: Neutrophil function: from mechanisms to disease. In: Annual Review of Immunology . Volume 30, 2012, pp. 459-489, ISSN 1545-3278 . doi: 10.1146 / annurev-immunol-020711-074942 . PMID 22224774 . (Review).
- ↑ Jutta Hein: Blood sampling and examination of small mammals. In: Kleintierpraxis 56 (2011), pp. 482–494.
- ↑ Lichtman, Andrew H., Pillai, Shiv, Preceded by: Abbas, Abul K .: Cellular and molecular immunology . Ed .: Abul K. Abbas. Ninth ed.Elsevier, Philadelphia, PA 2018, ISBN 978-0-323-52323-3 , pp. 608 .
- ^ A b c V. Brinkmann, U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, DS Weiss, Y. Weinrauch, A. Zychlinsky: Neutrophil extracellular traps kill bacteria. In: Science Volume 303, Number 5663, March 2004, pp. 1532-1535, ISSN 1095-9203 . doi: 10.1126 / science.1092385 . PMID 15001782 .
- ↑ Unknown weapon of our immune system discovered ( Memento from October 16, 2014 in the Internet Archive ) [], max-wissen.de
- ^ A b SR Clark, AC Ma, SA Tavener, B. McDonald, Z. Goodarzi, MM Kelly, KD Patel, S. Chakrabarti, E. McAvoy, GD Sinclair, EM Keys, E. Allen-Vercoe, R. Devinney, CJ Doig, FH Green, P. Kubes: Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. In: Nature Medicine . Volume 13, Number 4, April 2007, pp. 463-469, ISSN 1078-8956 . doi: 10.1038 / nm1565 . PMID 17384648 .
- ↑ CF Urban, U. Reichard, V. Brinkmann, A. Zychlinsky: Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. In: Cellular microbiology. Volume 8, Number 4, April 2006, pp. 668-676, ISSN 1462-5814 . doi: 10.1111 / j.1462-5822.2005.00659.x . PMID 16548892 .
- ↑ a b VS Baker, GE Imade, NB Molta, P. Tawde, SD Pam, MO Obadofin, SA Sagay, DZ Egah, D. Iya, BB Afolabi, M. Baker, K. Ford, R. Ford, KH Roux, TC Keller: Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. In: Malaria Journal . Volume 7, 2008, p. 41, ISSN 1475-2875 . doi: 10.1186 / 1475-2875-7-41 . PMID 18312656 . PMC 2275287 (free full text).
- ↑ A. Hakkim, BG Fürnrohr, K. Amann, B. Laube, UA Abed, V. Brinkmann, M. Herrmann, RE Voll, A. Zychlinsky: Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. In: Proceedings of the National Academy of Sciences . Volume 107, Number 21, May 2010, pp. 9813-9818, ISSN 1091-6490 . doi: 10.1073 / pnas.0909927107 . PMID 20439745 . PMC 2906830 (free full text).
- ^ AK Gupta, P. Hasler, W. Holzgreve, S. Gebhardt, S. Hahn: Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. In: Human immunology. Volume 66, Number 11, November 2005, pp. 1146-1154, ISSN 0198-8859 . doi: 10.1016 / j.humimm.2005.11.003 . PMID 16571415 .
- Jump up ↑ TA Fuchs, A. Brill, D. Duerschmied, D. Schatzberg, M. Monestier, DD Myers, SK Wrobleski, TW Wakefield, JH Hartwig, DD Wagner: Extracellular DNA traps promote thrombosis. In: Proceedings of the National Academy of Sciences . Volume 107, Number 36, September 2010, pp. 15880-15885, ISSN 1091-6490 . doi: 10.1073 / pnas.1005743107 . PMID 20798043 . PMC 2936604 (free full text).
- ^ A. Brill, TA Fuchs, AS Savchenko, GM Thomas, K. Martinod, SF De Meyer, AA Bhandari, DD Wagner: Neutrophil extracellular traps promote deep vein thrombosis in mice. In: Journal of thrombosis and haemostasis: JTH. Volume 10, Number 1, January 2012, pp. 136-144, ISSN 1538-7836 . doi: 10.1111 / j.1538-7836.2011.04544.x . PMID 22044575 . PMC 3319651 (free full text).
- ↑ JI Borissoff, H. ten Cate: From neutrophil extracellular traps release to thrombosis: an overshooting host-defense mechanism? In: Journal of thrombosis and haemostasis: JTH. Volume 9, Number 9, September 2011, pp. 1791-1794, ISSN 1538-7836 . doi: 10.1111 / j.1538-7836.2011.04425.x . PMID 21718435 .



