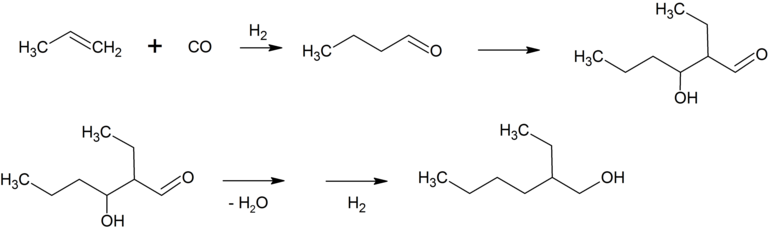Hydroformylation

The hydroformylation (also: oxo , rare Roelen synthesis or Roelen reaction ) is an industrially important, homogeneously catalyzed reaction of olefins with carbon monoxide and hydrogen . The primary products of hydroformylation are aldehydes with one more carbon atom than the olefin substrate. These aldehydes are used in the manufacture of a wide variety of useful derivatives, the aldehydes themselves are of poor utility. Important industrial products of hydroformylation are 1-butanol and 2-ethylhexanol , both of which are obtained from propene . The products of hydroformylation are used in a variety of ways as solvents or as intermediates for the production of detergents and cleaning agents , lubricants or plasticizers for plastics .
The German chemist Otto Roelen discovered this reaction in December 1937 while studying the Fischer-Tropsch synthesis in the Ruhrchemie research laboratories . The process is considered to be one of the most important developments in industrial chemistry of the 20th century and the first large-scale homogeneous catalytic process. The chemical industry uses organometallic cobalt or rhodium compounds as hydroformylation catalysts . The industrial process is carried out at pressures of around 10 bar to 100 bar and temperatures between 40 and 200 ° C. The capacity of the industrial plants has been continuously expanded since its invention, the total capacity of the hydroformylation plants is several million tons per year, with propene-based products making up the largest volume. Hydroformylation according to the two-phase Ruhrchemie / Rhône-Poulenc process based on water-soluble rhodium catalysts was one of the first technically important homogeneously catalyzed processes in which the crucial problem of recycling the catalyst was satisfactorily solved.
In addition to the industrial application possibilities, hydroformylation is an ideal atom- economical reaction for the formation of carbon-carbon bonds with unique possibilities for use in targeted organic synthesis. Through the use of catalyst systems with tailor-made ligands that control regio- , stereo- and enantioselectivity , hydroformylation became an important tool in the organic synthesis of fine chemicals.
history
In their investigations on the Fischer-Tropsch synthesis in 1926, OC Elvins and AW Nash first pointed out the formation of oxygen-containing components in this reaction. DF Smith, CO Hawk and PL Golden also observed the formation of oxygen-containing compounds during the reaction of ethylene with carbon monoxide and hydrogen under Fischer-Tropsch conditions. They received a mixture of aldehydes and alcohols, with a large part of the ethene used being hydrogenated to ethane . However, your attempts to optimize the process control with regard to oxygen-containing compounds were unsuccessful.
discovery
At the Ruhrchemie plant in Oberhausen , Otto Roelen discovered hydroformylation by chance in December 1937 while attempting to recycle the ethene produced in the Fischer-Tropsch synthesis into the process. In experiments in which ammonia was added to the Fischer-Tropsch synthesis in addition to ethene , Roelen found deposits of propionaldimine , a condensation product of ammonia and propionaldehyde . In contrast to other researchers, he interpreted the formation of propionaldehyde as an independent reaction, which he attributed to the addition of ethene and did not see it as a side reaction of the Fischer-Tropsch synthesis.
After initial attempts to optimize the reaction in the direction of aldehyde formation, which he began in July 1938, Roelen filed a patent for the oxo synthesis at the end of the same year. The name "oxo synthesis" is based on the false assumption that it was a general synthesis for the production of oxo products such as aldehydes and ketones. However, ketones are only obtained in large quantities in the hydroformylation of ethene as a secondary product in the form of diethyl ketone . Ruhrchemie, Otto Roelen's employer, chose toluene as the solvent for the hydroformylation , as it was easy to separate from the resulting products. At a reaction temperature of 115 ° C., with complete conversion of the ethene, the process achieved a yield of 70 to 80% propionaldehyde, 15% organic by-products and 5% loss.
Technical process
As a catalyst, Roelen used a catalyst containing cobalt oxide , thorium (IV) oxide and magnesium oxide , which was otherwise used for the Fischer-Tropsch synthesis. However, he found that many other cobalt salts were suitable as catalyst precursors and suggested that cobalt carbonyl hydride was the active catalyst species. This complex is considered to be the first generation of catalysts.
The IG Farben used a process with a variant on pumice fixed catalyst in an aqueous system. IG Farben used an equimolar mixture of ethene, carbon monoxide and hydrogen as input materials at reaction temperatures of 150 to 200 ° C and a reaction pressure of 15 to 30 MPa (150 to 300 bar). In this process variant, about 20 to 25% of the ethene was hydrogenated to ethane , the selectivity to propionaldehyde was only 65%. The loss of catalyst was compensated for by adding cobalt fatty acid salts.
Roelen worked very early on the hydroformylation of Fischer-Tropsch olefins with a chain length of 11 to 17 carbon atoms for the production of fatty alcohols . In 1940, Ruhrchemie began building a plant with an annual capacity of 7,000 tons of fatty alcohols. However, Ruhrchemie stopped operating the plant during the war.
Further developments
Due to the low selectivity for the n-isomer, Shell developed catalyst systems in which the carbon monoxide ligand of the cobalt carbonyl hydride was partially replaced by phosphines . By modifying the ligands it was now possible to partially control the selectivity for the desired product. The complexes of the HCo (CO) 3 (PR 3 ) type are considered to be the second generation of catalysts, which allowed process temperatures of 150 to 190 ° C. and pressures of 40 to 80 bar with an n / iso selectivity of 88:12.
Lauri Vaska reported in 1963 on the preparation of the complex rhodium tetracarbonyl hydride . Geoffrey Wilkinson began using this complex and its triphenylphosphine analogues for hydroformylation in 1968. This is considered a milestone in the further development of the process, since rhodium carbonyl hydride complexes with triphenylphosphine ligands have a high level of activity and selectivity in hydroformylation. The company Union Carbide used from 1976 catalysts of the type HRh (CO) (PR 3 ) 3 for the development of Low-Pressure-oxo process (LPO), which at temperatures of 90 to 100 ° C and pressures of 15 to 18 bar is working. Due to the low hydrogenation activity of the catalyst, milder reaction conditions and a high n / iso selectivity, the process quickly gained industrial importance despite the higher price of rhodium. Phosphine-modified rhodium carbonyl hydrides are the third generation of hydroformylation catalysts.
In addition to the original process, other companies such as BASF and Shell developed process variants that work at pressures of around 5 MPa (50 bar) and temperatures of around 100 ° C. The processes are all based on phosphane-modified cobalt and rhodium complexes. Shell used a tributylphosphine-modified cobalt carbonyl hydride for the hydroformylation of longer-chain olefins, which were further hydrogenated directly to form alcohol.
Two-phase hydroformylation
Numerous investigations have been carried out on fixing the catalyst with the aim of facilitating the separation of the product and catalyst phases. Attempts have been made, for example, to achieve fixation by combining diphenylphosphinomethylpolystyrene and dicobalt octacarbonyl . However, these efforts did not have the desired results. Problems with the deactivation of the catalyst and its discharge improved significantly in the 1980s with the introduction of water-soluble catalysts in the Ruhrchemie / Rhône-Poulenc process. Based on the rhodium triphenylphosphane carbonyl hydride developed by Wilkinson and the work of Kuntz at Rhône Poulenc, Ruhrchemie developed the technical process within 24 months and with a scale-up factor of 1: 24,000. The catalyst remains in the water phase, the lighter, water-insoluble product phase can be easily separated from the catalyst. The water-soluble rhodium catalysts are the fourth generation of hydroformylation catalysts.
Since the mid-1990s, attempts have been made to further optimize the reaction by using solvents such as supercritical carbon dioxide , perfluorinated systems or ionic liquids . In addition to the Ruhrchemie / Rhône-Poulenc process, other ways and methods of heterogenization were tested. In most cases, the problem of the catalyst discharge could not be solved. On August 24, 2013, on the 75th anniversary of the patent application, the Ruhrchemie plant was included by the GDCh in the Historic Chemical Sites program.
raw materials

Many olefins (alkenes and cycloalkenes) can be hydroformylated. Formally, it is an addition of hydrogen and a formyl group to the double bond of an olefin, the olefin / catalyst combination playing an essential role for the conversions and selectivities to be achieved.
Synthesis gas
In principle, synthesis gas can be produced from solid, liquid or gaseous starting materials. Both coal (in the formula example as C ) via coal gasification , crude oil ( C 5 H 12 ), natural gas ( CH 4 ) and renewable raw materials via steam reforming or partial oxidation can serve as carbon sources .
In 1986, Ruhrchemie put a coal gasification system into operation in the synthesis gas plant Ruhr based on the principle of a Texaco coal gasifier. A fine coal / water slurry was converted into synthesis gas at temperatures of around 1200 ° C. and a pressure of around 20 bar. The throughput was 30 tonnes of hard coal per hour, which resulted in 40,000 standard cubic meters of synthesis gas and 10,000 standard cubic meters of hydrogen.
In practice, a ratio of carbon monoxide to hydrogen of about 40:60 is desirable. The excess hydrogen is consumed in the parallel hydrogenation of the aldehydes to alcohols.
Olefins
Short-chain olefins usually react faster than longer-chain olefins and cycloolefins, linear olefins react faster than branched ones. Almost all olefins can be hydroformylated, the reaction of olefins which are fourfold substituted by alkyl groups and which would lead to a quaternary carbon atom, only rarely succeeding. Styrene can hardly be hydroformylated with cobalt catalysts, but high conversions are achieved with rhodium catalysts.
Propene , an unsaturated organic compound with the chemical formula C 3 H 6 , is by far the most common raw material for hydroformylation. Propene is obtained from fossil raw materials such as crude oil or natural gas and to a lesser extent from coal. Propene falls during cracking of naphtha and natural gas as a byproduct of processing. Another important source of petrochemical propene is the dehydrogenation of propane .
Conjugated dienes
Conjugated dienes can be hydroformylated to dialdehyde using rhodium-phosphine catalysts, while cobalt catalysts produce predominantly monoaldehydes by hydrogenating a double bond. Nonconjugated dienes can be hydroformylated to dialdehydes if the double bonds in the chain are separated by at least two carbon-carbon single bonds. Allyl alcohols, allyl esters and allyl ethers can preferably be hydroformylated using isomerization-free catalysts. α, β-unsaturated keto compounds usually react with hydrogenation of the double bond. Unsaturated carboxylic acids and carboxylic acid esters can be hydroformylated well, as can unsaturated aldehydes and ketones with non-conjugated double bonds.
Functionalized olefins
In addition to pure olefins, functionalized olefins such as allyl alcohol can be hydroformylated. The target product obtained with isomerization-free catalysts such as rhodium-triphenylphosphine complexes is 1,4-butanediol and its isomer. When using the cobalt complex, n - propanal is obtained by isomerization of the double bond . The hydroformylation of alkenyl ethers and alkenyl esters usually takes place in the α-position to the ether or ester function. The hydroformylated ester can lead to α, β-unsaturated aldehydes by subsequent cleavage of the carboxylic acid from the hydroformylation product.
Jürgen Falbe investigated the hydroformylation of acrylic acid and methacrylic acid . Then, in the first step of the rhodium-catalyzed variant, the Markovnikoff product is formed . By choosing the reaction conditions, the reaction can be steered in different directions. A high reaction temperature and low carbon monoxide pressures favor the isomerization of the Markovnikoff product to the thermodynamically more stable β-isomer, which leads to the n -aldehyde. Low temperatures, high carbon monoxide pressures and an excess of phosphines, which can occupy free coordination sites, lead to more rapid hydroformylation in the α-position to the ester group and suppress the isomerization.
The hydroformylation of conjugated olefins leads to the same products as the corresponding monoolefins with many catalyst systems by hydrogenating a double bond. With rhodium-phosphine complexes, hydroformylation leads to dialdehydes. The hydroformylation of alkynes leads to α, β-unsaturated aldehydes.
Catalysts
Metal carbonyl hydrides and their derivatives are used as hydroformylation catalysts . You can by the general formula
to be discribed. The catalysts used have a clearly defined structure and can be precisely characterized and synthesized with consistent quality or generated in-situ. In the catalytic process, all metal atoms are accessible as active centers for the synthesis of the product.
The chemical industry uses cobalt and rhodium complexes with various carbon monoxide, phosphine and phosphite ligands. Complexes with metals of the iron group such as iridium , iron , ruthenium and osmium as well as polymetallic systems such as platinum / tin were investigated, but did not show the same activity as rhodium and cobalt catalysts. Rhodium complexes are the most active hydroformylation catalysts and about 1000 times more active than cobalt complexes. The hydroformylation activity of the organometallic catalyst complex decreases from the cobalt complex to the iridium, ruthenium, osmium, manganese and iron complexes each time by a factor of about 10. Metallic calcium is also effective as hydroformylation catalysts; magnesium and zinc are less active . It is assumed that the activity of the metals can be traced back to the formation of hydrides. Only rhodium and cobalt complexes are important for industrial applications.
Cobalt catalysts
For the first hydroformylations, the Fischer-Tropsch synthesis catalyst was used, which consisted of about 30% cobalt, 2% thorium oxide , 2% magnesium oxide and 66% kieselguhr . This apparently heterogeneous catalyst was slurried along with the organic reactants. This slurry, also called mash, was then pumped into a high pressure chamber, where the hydroformylation took place under high pressure. After the reaction had ended, the now dissolved cobalt catalyst was reduced by hydrogen and adsorbed on the kieselguhr. Oil-soluble cobalt salts such as naphthenates were later used as catalyst precursors .
Under the reaction conditions, dicobalt octacarbonyl is initially formed from the cobalt salts or finely divided cobalt metal . The mononuclear complex cobalt carbonyl hydride (CoH (CO) 4 ) discovered by Walter Hieber , the actual catalyst for hydroformylation, is formed from this when hydrogen is absorbed .
Ligands
The ligand design has a great influence on the n - / iso ratio of the resulting products, especially through steric effects. Furthermore, the ratio of metal to ligand has an influence on the n- / iso-selectivity and the side reactions. Small additions of tertiary amines can accelerate the reaction; higher concentrations, however, can lead to the complete suppression of the reaction. When using rhodium as the catalyst metal, the catalytically active species is a trigonal-bipyramidal rhodium carbonylhydrido complex, which is present in two isomeric forms. The two phosphine ligands either occupy an equatorial-equatorial (ee) or the equatorial-apical (ea) position. The use of bidentate diphosphine ligands resulted in good selectivities for the linear aldehyde. The influence of the so-called bite angle of the diphosphine ligands was examined in detail.
In addition to the steric effects, the electronic effects of the ligand influence the catalyst activity. In rhodium complexes, good π acceptors such as phosphites lower the electron density on the metal through strong backbonding and accordingly weaken the rhodium-carbon monoxide bond. This facilitates the insertion of the carbon monoxide into the metal-alkyl bond. Organometallic rhodium phosphite complexes are therefore very good hydroformylation catalysts.
Reaction engineering
The technical processes differ according to the chain length of the olefin to be hydroformylated, the type of catalyst metal and the separation of the catalyst. The original Ruhrchemie process converted ethene into propanal using cobalt carbonyl hydride. Today, cobalt-based catalyst processes are mainly used for the production of medium to long-chain olefins, while rhodium-based catalysts are mostly used for the hydroformylation of propene. The rhodium catalysts are much more expensive than cobalt catalysts. In the hydroformylation of relatively high molecular weight olefins, loss-free separation from the catalyst is difficult. The processes differ mainly in the type of catalyst separation and the recovery of the catalyst.
Products
The primary products of hydroformylation are aldehydes, which are processed into a wide variety of secondary products by aldol condensation , hydrogenation , oxidation , amination and other processes. Over 70% of the total industrial production is accounted for by the production of n -butanal or n -butanol, around 20% by the production of C5 to C13 aldehydes, the remainder being made up of higher molecular weight aldehydes and propanal .
The further processing of the primarily formed aldehydes can take place in a two-stage sequence in which the hydrogenation takes place directly after the hydroformylation in a separate or in the same reaction vessel or by a hydroformylation under reducing conditions. Multi-stage further processing is also possible. The aldehydes initially react in a reaction such as aldol condensation to form secondary products before hydrogenation to alcohol takes place.
n -butanol
The butanal that is primarily formed in the hydroformylation of propene reacts with hydrogen further to form n-butanol. n -Butanol is an important intermediate in the production of butyl esters such as butyl acrylate , butyl acetate and dibutyl phthalate . n -Butanol is also used in the production of drugs, polymers, herbicides, catalysts and many other applications. Both n-butanol and isobutanol can be used pure or as an admixture to fuels for gasoline engines. These butanols have a higher energy density than ethanol.
2-ethylhexanol
The n- butanal primarily obtained in the hydroformylation of propene reacts under basic catalysis to form 2-ethylhexenal , which is converted to 2-ethylhexanol (2-EH) by dehydration and subsequent hydrogenation .
This alcohol is reacted with phthalic anhydride to form bis (2-ethylhexyl) phthalate , which is simply referred to as dioctyl phthalate (DOP). Bis (2-ethylhexyl) phthalate is an important plasticizer for polyvinyl chloride (PVC). Around 2.5 million tons of bis (2-ethylhexyl) phthalate are produced annually. About 25% of the n -butanal is hydrogenated to n-butanol , which is used as a solvent and for esterifications . The higher molecular weight aldehydes, which are obtained, for example, by hydroformylation of SHOP olefins with cobalt catalysts, are usually hydrogenated to fatty alcohols. These are sulfated , often after ethoxylation , and used as anionic surfactants after neutralization with caustic soda or ammonia .
Laboratory syntheses
Prochiral olefins can be hydroformylated enantioselectively with chiral complexes . For example, dexibuprofen , the (+) - ( S ) -enantiomer of ibuprofen , can be produced by enantioselective hydroformylation and subsequent oxidation . By using chiral phosphine ligands such as DIOP , DIPAMP or BINAPHOS , high enantiomeric excess can be achieved. Asymmetric hydroformylation is used, among other things, to synthesize chiral products that are used in pharmacy or as active components for agrochemicals . In contrast to technical hydroformylation, the target product is the branched aldehyde with a center of chirality .
Only the product of the Markovnikov addition leads to the target product, while the n -aldehyde is achiral. The stereochemistry is determined in the olefin coordination step.
The hydroformylation can be modified to what is known as silylformylation by replacing hydrogen with monohydrosilanes (H-Si-R 3 ) . A trialkylsilyl group and a formyl group are added to the triple bond of an alkyne and form a 3-silyl-2-alkenal.
The tandem reaction of hydroformylation with, for example, Knoevenagel reactions , Wittig olefinations or allylborations enables the construction of complex molecules, some of which can be carried out in a one-pot reaction , for example in combination with a reductive amination as hydroaminomethylation .
Reaction mechanism
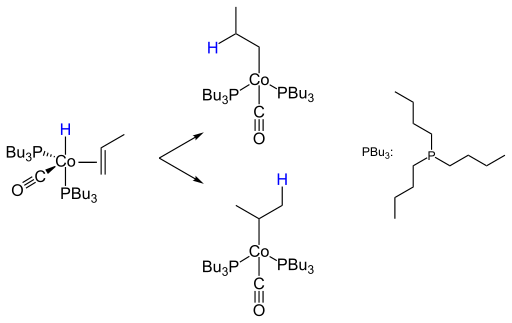
The so-called Heck-Breslow mechanism of cobalt-catalyzed hydroformylation was elucidated in 1960 by the later Nobel Prize winners Richard F. Heck and David Breslow. According to this, a carbon monoxide ligand is first eliminated from cobalt carbonyl hydride with the formation of a 16-electron species ( 1 ). This creates a free coordination site to which an olefin can attach itself by means of a π bond to form an 18-electron species ( 2 ). The next step is the formation of a 16-electron alkyl complex, in whose free coordination site a carbon monoxide ligand is incorporated ( 3 ). This in turn inserts into the metal-carbon bond of the alkyl radical to form a 16-electron acyl complex ( 4 ). Oxidative addition of hydrogen releases the aldehyde and restores the active species ( 5 ). The catalytic cycle closes with the formation of the starting complex. As a side reaction, the 16-electron complex can take up a molecule of carbon monoxide in an equilibrium reaction ( 6 ). In both cobalt and rhodium-catalyzed hydroformylation, the oxidative addition of hydrogen is the rate-determining step with subsequent reductive elimination of the aldehyde.
Rhodium hydridocarbonyls and their phosphine-modified analogues react according to a similar mechanism, which Geoffrey Wilkinson investigated in 1968 . In the first step, a phosphine ligand dissociates from the complex and forms a planar, coordinatively unsaturated 16-electron species. An olefin coordinates to this to form an 18-electron complex. After the olefin has been inserted into the rhodium-hydrogen bond with formation of an alkyl complex and after the addition of a further molecule of carbon monoxide, this inserts into the rhodium-alkyl bond with the formation of the acyl complex. With the addition of carbon monoxide, the catalytic cycle closes and the original complex is restored.
The formation of the square-planar intermediate stage with two sterically demanding phosphine ligands is considered to explain the high n / iso ratio of the rhodium-catalyzed hydroformylation. The steric constraints in the transition state mean that the alkyl ligand is preferably coordinated linearly. This explains why the n / iso ratio is positively influenced by increasing phosphine concentration and decreasing carbon monoxide partial pressure.
The mechanism was investigated using infrared spectroscopic and high pressure NMR methods. The use of deuterium in the reaction (deuteroformylation) allows the products formed to be examined by means of 1 H-NMR spectroscopy and thus conclusions about the mechanism.
Regioselectivity
An important criterion in hydroformylation is the regioselectivity to the n- or iso-product. The n-isomer of propene hydroformylation is processed further to 2-ethylhexanol on an industrial scale , whereas the iso- isomer is only of secondary importance for the preparation of isobutanol , neopentyl glycol or isobutyric acid . The step of insertion of the olefin into the metal-hydrogen bond to form of the alkyl decides over the selectivity of formation of n - or iso aldehydes:
In the case of higher molecular weight olefins, isomerization of the double bond can occur, as a result of which mixtures of aldehydes are formed. A carbon monoxide ligand inserts into the metal-carbon bond of the alkyl complex to form an acyl complex.
Keulemans set 1948 rules of the product distribution ( Keulemans rules ) Accordingly, from straight chain olefins is always a mixture of n- and 2-alkyl alcohols in the ratio of 40 to 60% n - and 60 to 40% 2-alkyl alcohols. There is no addition of the formyl group to tertiary carbon atoms; For example, isobutene only forms 3-methylbutanol. The addition of carbon atoms in the α-position to tertiary carbon atoms is sterically hindered, but can take place. There is no addition of carbon atoms in the α-position to quaternary carbon atoms. An isolated tertiary carbon atom hinders the formation of the possible isomers. The hydroformylation is always accompanied by a double bond isomerization. Apart from the 2-alkyl branching, there is no tendency to increase the degree of branching. A linear product is preferably obtained with a shift in the double bond.
Kinetic investigations yielded the following rate equation for the cobalt-catalyzed reaction:
The reaction is exothermic by about 125 kJ mol −1 . In rhodium-catalyzed hydroformylations, turnover numbers of about 6000 mol olefin mol catalyst −1 h −1 are achieved.
Side and follow-up reactions
Reactions of olefins
Side reactions of the olefins are the isomerization and hydrogenation of the olefinic double bond . While the alkanes formed by hydrogenation of the double bond no longer participate in the reaction, the isomerization of the double bond with subsequent formation of the n -alkyl complexes is a desired process. The hydrogenation is usually of minor importance. Cobalt phosphine-modified catalysts, however, can have increased hydrogenation activity, with up to 15% of the olefin being hydrogenated.
Reactions of aldehydes
A mostly desired side reaction is the hydrogenation of the aldehydes to alcohols. Higher temperatures and hydrogen partial pressures favor the hydrogenation of the aldehydes formed to alcohol. The kinetics of alcohol formation with cobalt complexes can be described with the following equation:
The reaction mechanism is assumed to be that the π-complex of the aldehyde and the catalyst is initially formed. With rearrangement to the alcoholate and subsequent oxidative addition of hydrogen, the alcohol and the starting complex are formed:
The aldehydic carbon-oxygen double bond can also undergo hydroformylation and lead to formic acid and its esters. Carbon monoxide is inserted into the oxygen-metal bond. The resulting formyl complex can release the formic acid ester with the oxidative addition of hydrogen:
The primarily formed aldehydes can also react further and, through aldol condensation, form products such as the target product precursor 2-ethylhexenal or higher molecular weight condensation products, so-called thick oil.
Homologation of the alcohols
Under the reaction conditions of the hydroformylation, the alcohols formed can be dehydrated again to the olefin using an acidic catalyst such as cobalt carbonyl hydride. A further hydroformylation of the olefin leads to chain extension and formation of an aldehyde. This is again reduced to alcohol, which in turn can be dehydrated olefin. The willingness to react is greatest with tertiary alcohols, secondary alcohols react more slowly and primary alcohols react slowly. Of tert -butanol as arises isovaleraldehyde . Even methanol and benzyl alcohol can be homologated to ethanol or 2-phenylethanol .
Reactions of the catalyst complex
The triphenylphosphine complexes used can liberate benzene by hydrogenation under the reaction conditions. The insertion of carbon monoxide into an intermediate metal-carbon bond can lead to the formation of benzaldehyde or, through subsequent hydrogenation, to benzyl alcohol . The ligand can add propene, and the resulting diphenylpropylphosphine can inhibit the reaction due to its increased basicity.
Trace contamination of the starting materials with oxygen or sulfur and their compounds can lead to the oxidation of phosphorus (III) to phosphorus (V) compounds or to catalytically inactive metal oxides and sulfides .
Process variants
Ruhrchemie / Rhône-Poulenc process
In the Ruhrchemie / Rhône-Poulenc process, a rhodium complex (Kuntz- Cornils catalyst) complexed with triphenylphosphine trisulfonate (TPPTS) is used as the catalyst . By substituting the triphenylphosphine ligand with sulfonate groups, the organometallic complex has hydrophilic properties. As a result of the nine- fold sulfonation, the catalyst is very soluble in water (about 1 kg l −1 ), but not in the resulting product phase. The water-soluble triarylphosphine sulfonate is used in an approximately 50-fold excess, which effectively suppresses the washing out of the catalyst, the so-called "leaching". The starting materials used are propene and synthesis gas, which consists of hydrogen and carbon monoxide in a ratio of 1.1: 1. It can be obtained from various raw material sources independent of petroleum . The product is a mixture of n - and iso - butanal in a ratio of 96: 4. The selectivity for n -aldehyde is high, by-products such as alcohols, esters and higher-boiling fractions are hardly formed.
The Ruhrchemie / Rhône-Poulenc process is the first commercialized two-phase system in which the catalyst is in an aqueous phase. In the course of the reaction, an organic product phase is formed which is continuously separated off by means of phase separation, the aqueous catalyst phase remaining in the reactor.
In this process, the olefin and the synthesis gas are fed into the reactor from below in a stirred tank reactor and the phases of the reaction mixture are intensively mixed. The resulting crude aldehyde is drawn off at the top. The organic phase is separated from the aqueous phase in the phase separator. The aqueous catalyst-containing solution is preheated via a heat exchanger and pumped back into the reactor. In a stripper, the excess olefin is separated from the organic phase by synthesis gas in the absence of a catalyst and fed back into the reactor. The released heat of reaction is used to generate process steam via heat exchangers.
The process steam generated is used for the subsequent distillation of the organic phase to separate iso - and n - butanal. The distillation bottom is heated by a falling film evaporator and returned to the distillation. Potential catalyst poisons that are introduced into the reaction via the synthesis gas are separated off with the aldehyde. As a result, there is no accumulation of catalyst poisons, so the time-consuming fine cleaning of the synthesis gas can be omitted.
A plant was built in Oberhausen in 1984, which was expanded in 1988 and again in 1998 to a production capacity of 500,000 t / year of butanal. 98% of the propene is converted and a high selectivity is achieved. Less than 1 ppb of rhodium is lost during the process.
BASF process
In the hydroformylation process at BASF (BASF Oxo process), higher olefins are mostly used. Cobalt carbonyl hydride serves as the catalyst. The catalyst is oxidized by oxygen from the formally negatively charged Co −1 to the water-soluble Co 2+ in the liquid product phase and separated by adding aqueous formic or acetic acid . This forms an aqueous phase that contains the catalyst metal in the form of its salt. The aqueous phase is separated off and the cobalt is returned to the process. Any losses are compensated for by adding fresh cobalt salts. A reaction at low temperature leads to an increased selectivity to the linear product. The process is carried out at a pressure of about 30 MPa and in a temperature range from 150 to 170 ° C.
Exxon process
The Exxon process or Kuhlmann or PCUK Oxo process is used for the hydroformylation of C6 to C12 olefins. To recover the catalyst, aqueous sodium hydroxide or sodium carbonate solution is added to the organic product phase. The metal carbonyl hydride is recovered by extraction with olefin and neutralization by adding sulfuric acid solution under carbon monoxide pressure. This is stripped out with synthesis gas, absorbed by the olefin and returned to the reactor. The process is started at a pressure of about 30 MPa and at a temperature of about 160 to 180 ° C.
Shell procedure
The Shell process uses cobalt complexes with phosphine ligands for the hydroformylation of C7 to C14 olefins. The resulting aldehydes are further hydrogenated directly to the fatty alcohol. These are distilled off from the catalyst overhead and the catalyst is obtained as a bottom product and can be returned to the process. The process has a good selectivity for linear products that are used as surfactant raw materials. It is carried out at a pressure of about 4 to 8 MPa and in a temperature range of about 150 to 190 ° C.
UCC procedure
The UCC process, also known as the Low-Pressure-Oxo-Process (LPO), uses a rhodium catalyst dissolved in high-boiling thick oil for the hydroformylation of propene. The reaction mixture is separated from volatile constituents in a falling film evaporator. The liquid phase is distilled and n- butanal is separated overhead from the thick oil originating from the catalyst phase . In this process a pressure of about 1.8 MPa is used in a temperature range of about 95-100 ° C.
literature
- Piet WNM van Leeuwen, Carmen Claver: Rhodium Catalyzed Hydroformylation. Verlag Springer Netherlands, 2000, ISBN 0-7923-6551-8 .
- Arno Behr : Applied Homogeneous Catalysis. Wiley-VCH, 2008, ISBN 978-3-527-31666-3 .
- Boy Cornils : hydroformylation (oxo synthesis). In: J. Falbe, U. Hasserodt: catalysts, tensides and mineral oil additives , Georg Thieme Verlag, 1978, ISBN 3-13-552601-1 .
- Armin Börner, Robert Franke: Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis , Wiley-VCH, 2016, ISBN 978-3-527-33552-7
Web links
Individual evidence
- ^ OC Elvins, AW Nash: Synthetic Fuel From Carbon Monoxide and Hydrogen. In: Fuel , 5, 1926, pp. 263-265.
- ↑ David F. Smith, Charles O. Hawk, Paul L. Golden: The Mechanism of the Formation of Higher Hydrocarbons from Water Gas . In: Journal of the American Chemical Society. 52, 1930, pp. 3221-3232, doi : 10.1021 / ja01371a030 .
- ↑ a b c d Boy Cornils, Wolfgang A. Herrmann, Manfred Rasch: Otto Roelen as a trailblazer for industrial homogeneous catalysis. In: Angewandte Chemie. 106, 1994, pp. 2219-2238, doi : 10.1002 / ange.19941062104 .
- ↑ Historic sites of chemistry: Otto Roelen , Oberhausen, September 24, 2013.
- ↑ a b c B. IOS - Final Report No. 447, Item No. 30: Interrogation of Dr. Otto Roelen of Ruhrchemie AG (No longer available online.) Archived from the original on February 8, 2012 ; Retrieved June 14, 2012 .
- ^ SS Bath, L. Vaska: Five-Coordinate Hydrido-Carbonyl Complexes of Rhodium and Iridium and their Analogy with CoH (CO) 4 . In: Journal of the American Chemical Society. 85, 1963, pp. 3500-3501, doi : 10.1021 / ja00904a044 .
- ↑ D. Evans, JA Osborn, G. Wilkinson: Hydroformylation of alkenes by use of rhodium complex catalysts. In: Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1968, p. 3133, doi : 10.1039 / J19680003133 .
- ↑ CK Brown, G. Wilkinson: Homogeneous hydroformylation of alkenes with hydridocarbonyltris (triphenylphosphine) rhodium (I) as catalyst. In: Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1970, pp. 2753-2764, doi : 10.1039 / J19700002753 .
- ^ Anna M. Trzeciak, Józef J. Ziółkowski: Perspectives of rhodium organometallic catalysis. Fundamental and applied aspects of hydroformylation. In: Coordination Chemistry Reviews. 190-192, 1999, pp. 883-900, doi : 10.1016 / S0010-8545 (99) 00127-7 .
- ↑ a b c Jens Weitkamp , Roger Gläser: Catalysis. In: Winnacker / Küchler. Chemical engineering: processes and products. Edited by Roland Dittmeyer, Wilhelm Keim , Gerhard Kreysa , Alfred Oberholz, Volume 1: Methodical Basics. Wiley-VCH Verlag, Weinheim 2004, ISBN 3-527-30767-2 , pp. 47-49.
- ↑ Boy Cornils : Hydroformylation (Oxo synthesis). In: J. Falbe, U. Hasserodt: Catalysts, Tenside und Mineralöladditive , Georg Thieme Verlag, 1978, ISBN 3-13-552601-1 , pp. 108-111.
- ↑ S. Kanagasabapathy, Zhigao Xia, Georgios Papadogianakis, Bernhard Fell : Hydroformylation with water- and methanol-soluble rhodium carbonyl / phenyl-sulfonatoalkylphosphine catalyst systems - a new concept for the hydroformylation of higher molecular weight olefins. In: Journal for practical chemistry / Chemiker-Zeitung. 337, 1995, pp. 446-450, doi : 10.1002 / prac.19953370197 .
- ^ A b Boy Cornils, Wolfgang A. Herrmann: Aqueous-Phase Organometallic Catalysis, Concepts and Applications. Wiley-VCH Verlag, 1998, ISBN 3-527-29478-3 , pp. 271-273.
- ↑ Marco Haumann, Anders Riisager: Hydroformylation in Room Temperature Ionic Liquids (RTILs): Catalyst and Process Developments. In: Chemical Reviews. 108, 2008, pp. 1474-1497, doi : 10.1021 / cr078374z .
- ↑ Renate Hoer: Otto Roelen and Ruhrchemie. In: gdch.de. January 22, 2014, accessed November 19, 2014 .
- ^ Sieghard Neufeldt: Chronologie Chemie, Verlag Wiley-VCH, 2003, ISBN 3-527-29424-4 , p. 339.
- ↑ CK Brown, G. Wilkinson: Homogeneous hydroformylation of alkenes with hydridocarbonyltris (triphenylphosphine) rhodium (I) as catalyst. In: Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1970, pp. 2753-2764, doi: 10.1039 / J19700002753 .
- ↑ JH Jones, TE Daubert, MR Fenske: Oxidation and Oxidative Dehydrogenation of Ethane and Propane. In: Industrial & Engineering Chemistry Process Design and Development. 8, 1969, pp. 17-25, doi: 10.1021 / i260029a004 .
- ↑ PWNM Van Leeuwen, CF Roobeek: The hydroformylation of butadiene catalysed by rhodium-diphosphine complexes. In: Journal of Molecular Catalysis. 31, 1985, pp. 345-353, doi: 10.1016 / 0304-5102 (85) 85117-8 .
- ↑ Bernhard Fell, Wolfgang Rupilius, Friedrich Asinger : On the question of isomer formation in the hydroformylation of higher molecular weight olefins with complex cobalt and rhodium catalysts. In: Tetrahedron Letters 9, 1968, pp. 3261-3266, doi : 10.1016 / S0040-4039 (00) 89542-8 .
- ↑ a b Jürgen Falbe, Ch. R. Adams: Carbon Monoxide in Organic Synthesis. Springer Verlag, 1970, ISBN 3-540-04814-6 .
- ↑ Bernhard Fell , Wolfgang Rupilius: Dialdehydes by hydroformylation of conjugated dienes. In: Tetrahedron Letters. 10, 1969, pp. 2721-2723, doi : 10.1016 / S0040-4039 (01) 88252-6 .
- ↑ John R. Johnson, Gregory D. Cuny, Stephen L. Buchwald: Rhodium-Catalyzed Hydroformylation of Internal Alkynes to α, β-Unsaturated Aldehydes. In: Angewandte Chemie. International edition in English. 34, 1995, pp. 1760-1761, doi : 10.1002 / anie.199517601 .
- ↑ Georg Süss-Fink , Josel Reiner: The cluster anion [HRu3 (CO) 11] - as catalyst in hydroformylation, hydrogenation, silacarbonylation and hydrosilylation reactions of ethylene and propylene. In: Journal of Molecular Catalysis. 16, 1982, pp. 231-242, doi : 10.1016 / 0304-5102 (82) 85011-6 .
- ^ B. Cornils, WA Herrmann, R. Schlögl, CH Wong: Catalysis from A to Z. Verlag Wiley-VCH, 2000, ISBN 3-527-29855-X .
- ^ E. Müller, O. Bayer, H. Meerwein, K. Ziegler: Houben-Weyl Methods of Organic Chemistry Vol. VII / 1, 4th Edition: Aldehydes , Georg Thieme Verlag, Stuttgart, 1975, ISBN 978-3-13- 205704-3 , p. 59.
- ↑ Patent US3239569A : Hydroformylation of olefins. Published May 13, 1963 , Inventors: Lynn H. Slaugh, Richard D. Mullineaux.
- ↑ Christoph Elschenbroich: Organometallchemie. 6th edition. Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 633-637.
- ↑ W. Hieber, F. Mühlbauer, EA Ehmann: Derivate des Kobalt- und Nickelcarbonyls (XVI. Communication. About metal carbonyls). In: Reports of the German Chemical Society. 65, 1932, pp. 1090-1101, doi: 10.1002 / cber.19320650709 .
- ↑ TJ Devon et al. a .: Chelate ligands for low pressure hydroformylation catalyst and process employing same, US 4694109, Sep. 15, 1987, Eastman Kodak Company.
- ↑ Piet WNM van Leeuwen, Zoraida Freixa: Bite Angle Effects of Diphosphines in Carbonylation Reactions. In: László Kollár: Modern Carbonylation Methods , Verlag Wiley-VCH, Weinheim, ISBN 978-3-527-31896-4 .
- ↑ a b c d e Dirk Steinborn: Fundamentals of Organometallic Catalysis. Wiley-VCH Verlag, 2011, ISBN 3-527-32717-7 .
- ↑ C. Kohlpaintner, M. Schulte, J. Falbe, P. Lappe, J. Weber: aldehyde, Aliphatic. In: Ullmann's Encyclopedia of Industrial Chemistry 2008 , Wiley-VCH, Weinheim. doi : 10.1002 / 14356007.a01_321.pub2 .
- ↑ a b c Arno Behr : Applied homogeneous catalysis , Wiley-VCH. Weinheim, ISBN 3-527-31666-3 .
- ↑ Kyoko Nozaki, Nozomu Sakai, Tetsuo Nanno, Takanori Higashijima, Satoshi Mano, Toshihide Horiuchi, Hidemasa Takaya: Highly Enantioselective Hydroformylation of Olefins Catalyzed by Rhodium (I) Complexes of New Chiral Phosphine / Phosphite Ligands. In: Journal of the American Chemical Society. 119, 1997, pp. 4413-4423, doi : 10.1021 / ja970049d .
- ↑ Carlo Botteghi, Stefano Paganelli, Alberto Schionato, Mauro Marchetti: The asymmetric hydroformylation in the synthesis of pharmaceuticals. In: Chirality. 3, 1991, pp. 355-369, doi : 10.1002 / chir.530030422 .
- ^ Francine Agbossou, Jean-Francois Carpentier, Andre Mortreux: Asymmetric Hydroformylation. In: Chemical Reviews. 95, 1995, pp. 2485-2506, doi : 10.1021 / cr00039a008 .
- ↑ Hiromi Okazaki, Yukio Kawanami, Keiji Yamamoto: The Silyl Formylation of Simple 1-Alkynes Catalyzed by [Rh (cod)] [BPh4] in an Ionic Liquid, [Bmim] [PF6], under Biphasic Conditions: An Efficiently Reusable Catalyst System. .. In: Chemistry Letters. 2001, pp. 650-651, doi : 10.1246 / cl.2001.650 .
- ↑ Delphine Crozet, Martine Urrutigo ty, Philippe Kalck: Recent Advances in Amine Synthesis by Catalytic Hydroaminomethylation of Alkenes. In: ChemCatChem. 3, 2011, pp. 1102-1118, doi : 10.1002 / cctc.201000411 .
- ^ A b Richard F. Heck , David S. Breslow: The Reaction of Cobalt Hydrotetracarbonyl with Olefins. In: Journal of the American Chemical Society. 83, 1961, pp. 4023-4027, doi : 10.1021 / ja01480a017 .
- ^ Jack Halpern: Organometallic chemistry at the threshold of a new millennium. Retrospect and prospect. (PDF; 131 kB) In: Pure Appl. Chem. , 2001, vol. 73, no. 2, pp. 209-220.
- ^ Marc Garland, Gyorgy Bor: Infrared spectroscopic studies on metal carbonyl compounds. 24. Observation of the infrared spectrum of an acylrhodium tetracarbonyl during the hydroformylation of olefins with rhodium-containing catalyst precursors. In: Inorganic Chemistry. 28, 1989, pp. 410-413, doi : 10.1021 / ic00302a008 .
- ↑ Claudio Bianchini, Hon Man Lee, Andrea Meli, Francesco Vizza: In Situ High-Pressure 31 P { 1 H} -NMR Studies of the Hydroformylation of 1-Hexene by RhH (CO) (PPh 3 ) 3 . In: Organometallics. 19, 2000, pp. 849-853, doi : 10.1021 / om9907627 .
- ↑ Raffaello Lazzaroni, Andrea Raffaelli, Roberta Settambolo, Sergio Bertozzi, Giovanni Vitulli: Regioselectivity in the rhodium-catalyzed hydroformylation of styrene as a function of reaction temperature and gas pressure. In: Journal of Molecular Catalysis. 50, 1989, pp. 1-9, doi : 10.1016 / 0304-5102 (89) 80104-X .
- ↑ AIM Keulemans, A. Kwantes, Th. Van Bavel: The structure of the formylation (OXO) products obtained from olefines and watergas. In: Recueil des Travaux Chimiques des Pays-Bas. 67, 1948, pp. 298-308, doi : 10.1002 / recl.19480670406 .
- ↑ G. Natta, R. Ercoli, S. Castellano, FH Barbieri: The Influence of Hydrogen and Carbon Monoxide Partial Pressures on the Rate of the Hydroformylation Reaction. In: Journal of the American Chemical Society. 76, 1954, pp. 4049-4050, doi : 10.1021 / ja01644a071 .
- ↑ G. Natta: Oxo synthesis, its kinetics and related reactions. In: Fuel Chemistry , No. 11/12, Vol. 36, June 1, 1955, pp. 176-181.
- ↑ a b c d e Boy Cornils , Wolfgang A. Herrmann , Chi-Huey Wong, Horst -Werner Zanthoff: Catalysis from A to Z: A Concise Encyclopedia. Verlag Wiley-VCH Verlag, 2012, ISBN 3-527-33307-X .
- ↑ Detlef Selent, Dieter Hess, Klaus-Diether Wiese, Dirk Röttger, Christine Kunze, Armin Börner: Rhodium-catalyzed isomerization / hydroformylation of internal octenes with novel phosphorus ligands. In: Angew. Chem. 113, 2001, p. 1739, doi : 10.1002 / 1521-3757 (20010504) 113: 9 <1739 :: AID-ANGE17390> 3.0.CO; 2-5 .
- ↑ Irving Wender, Robert Levine, Milton Orchin: Homologation of Alcohols. In: Journal of the American Chemical Society. 71, 1949, pp. 4160-4161, doi: 10.1021 / ja01180a520 .
- ^ I. Wender, RA Friedel, M. Orchin: Ethanol from methanol. In: Science. 113, 1951, pp. 206-207, doi: 10.1126 / science.113.2930.206 .
- ↑ Wolfgang A. Herrmann, Christian W. Kohlpaintner: Water-soluble ligands, metal complexes and complex catalysts: Synergisms from homogeneous and heterogeneous catalysis. In: Angewandte Chemie. 105, 1993, pp. 1588-1609 doi: 10.1002 / anie.19931051102 .
- ↑ a b c d e f g Ernst Wiebus, Boy Cornils: The large-scale oxo synthesis with immobilized catalyst. In: Chemical Engineer Technology. 66, 1994, pp. 916-923, doi : 10.1002 / cite.330660704 .
- ^ W. Keim : C1 Chemistry: potential and developments. In: Pure & AppL Chem. , Vol. 58, No. 6, pp. 825-832, 1986 ( PDF ( memento of October 29, 2013 in the Internet Archive )).
- ^ A b Manfred Baerns , Arno Behr , Axel Brehm, Jürgen Gmehling , Hanns Hofmann , Ulfert Onken : Technical Chemistry Textbook . 480 figures, 190 tables. Wiley-VCH-Verlag, 2006, ISBN 3-527-31000-2 .
- ↑ G. Duembgen, D. Neubauer: Large-scale production of oxo alcohols from propylene in BASF. In: Chemical Engineer Technology - CIT. 41, 1969, pp. 974-980, doi : 10.1002 / cite.330411708 .








![{\ displaystyle \ mathrm {[Co_ {2} (CO) _ {8}] \ + \ H_ {2} \ longrightarrow \ 2 \ [CoH (CO) _ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a4f80426e35289df4aa70d29aa181a9541c9ca69)