Isovaleraldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
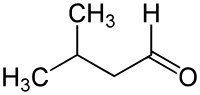
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isovaleraldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 10 O | |||||||||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 86.13 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.80 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−51 ° C |
|||||||||||||||
| boiling point |
92 ° C |
|||||||||||||||
| Vapor pressure |
61 hPa (20 ° C) |
|||||||||||||||
| solubility |
poor in water (20 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.3902 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Isovaleraldehyde is a chemical compound from the group of aldehydes . With its isomeric compounds valeraldehyde ( n- pentanal), 2-methylbutyraldehyde and pivalaldehyde , it forms the substance group of pentanals .
Occurrence
Isovaleraldehyde occurs as a component of the alarm pheromone in the wasp Vespula vulgaris .
Extraction and presentation
Isovaleraldehyde is produced by the hydroformylation of isobutene .
In 2000 several thousand tons were produced in Germany. This makes it one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible dangers (" Screening Information Dataset ", SIDS).
properties
Isovaleraldehyde is sensitive to light and air and has a viscosity of 0.58 mPas at 20 ° C.
use
Isovaleraldehyde is used as an intermediate in the manufacture of drugs, vitamins , pesticides , solvents and plasticizers .
safety instructions
The vapors of isovaleraldehyde can form an explosive mixture with air ( flash point −3 ° C, ignition temperature 210 ° C).
proof
Isovaleraldehyde can be detected by absorption with a 2,4-dinitrophenylhydrazine impregnated glass fiber filter, subsequent desorption with acetonitrile, followed by chromatographic determination by HPLC with a UV detector (365 nm).
Individual evidence
- ↑ a b c d e f g h i j Entry on isovaleraldehyde in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-342.
- ↑ Cosmetic products / alarm pheromones from hornets and wasps. (PDF; 87 kB) Cantonal Laboratory Basel, September 21, 2004, accessed on August 22, 2017 .
- ↑ a b Justification on isovaleraldehyde in TRGS 900
- ^ Fischer-Tropsch.org: Ammoniaklaboratorium Oppau, report no. 2001, 2.1.1946 by Dr. Nienburg, Oxo-Arbeit 1940-1944 ( Memento from August 31, 2011 in the Internet Archive ) (PDF; 1.6 MB).
- ↑ a b OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for butanal, 3-methyl- , accessed on November 4, 2014.


