Pentanals
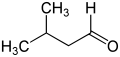
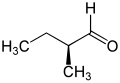
The pentanals are a group of four isomeric saturated aldehydes with five carbon atoms. They have the general empirical formula C 5 H 10 O and a molar mass of 86.13 g / mol. They can also be thought of as being composed of a butyl and an aldehyde group (-CHO); this means that all four variants ( structural isomers ) are represented, some of which differ significantly in their properties. 2-methylbutyraldehyde is chiral and also forms two stereoisomeric forms.
You are u. a. accessible by oxidation of the corresponding primary pentanols ( 1-pentanol , 3-methyl-1-butanol , 2-methyl-1-butanol and 2,2-dimethyl-1-propanol ). The analogous pentanoic acids are formed by further oxidation .
The pentanones have the same molar mass.
| Pentanals | |||||
| Surname | Valeraldehyde | Isovaleraldehyde | 2-methylbutyraldehyde | Pivalaldehyde | |
| other names | n -pentanal | iso pentanal | sec pentanal | tert -pentanal | |
| ( S ) -2-methylbutyraldehyde | ( R ) -2-methylbutyraldehyde | ||||
| Structural formula |  |
 |
 |
 |

|
| CAS number | 110-62-3 | 590-86-3 | 96-17-3 ( racemate ) | 630-19-3 | |
| 1730-97-8 | 33204-48-7 | ||||
| PubChem | 8063 | 11552 | 7284 (racemate) | 12417 | |
| 6971248 a | |||||
| Melting point | −92 ° C | −51 ° C | <−60 ° C (racemate) | 6 ° C | |
| boiling point | 103 ° C | 92 ° C | 91-93 ° C (racemate) | 74 ° C (973 hPa) | |
|
a Note: The structural formula shown at PubChem has the wrong stereochemistry
|
|||||
IUPAC nomenclature
According to the IUPAC nomenclature, strictly speaking, only n -pentanal is a pentanal. 3-methyl-1-butanal, 2-methyl-1-butanal, and 2,2-dimethyl-1-propanal are therefore not pentanals.
Individual evidence
- ↑ a b Entry for CAS no. 110-62-3 in the GESTIS substance database of the IFA , accessed on December 1, 2012(JavaScript required) .
- ↑ a b Entry for CAS no. 590-86-3 in the GESTIS substance database of the IFA , accessed on December 1, 2012(JavaScript required) .
- ↑ a b Entry for CAS no. 96-17-3 in the GESTIS substance database of the IFA , accessed on December 1, 2012(JavaScript required) .
- ↑ a b Data sheet trimethylacetaldehyde from Sigma-Aldrich , accessed on December 1, 2012 ( PDF ).