Neopentyl glycol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Neopentyl glycol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 5 H 12 O 2 | ||||||||||||||||||
| Brief description |
white, sweet-smelling solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 104.15 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.06 g cm −3 |
||||||||||||||||||
| Melting point |
128 ° C |
||||||||||||||||||
| boiling point |
208 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Neopentyl glycol (NPG for short) is a fully synthetic , branched primary and polyhydric alcohol with a sweet smell. Due to its excellent properties in terms of chemical and thermal stability, it is the starting material for numerous applications.
Extraction and presentation
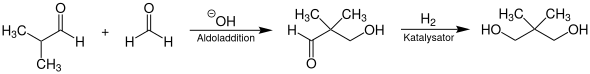
Neopentyl glycol is produced on an industrial scale by aldol addition of isobutyraldehyde and formaldehyde in the presence of a tertiary alkylamine as a catalyst . Triethylamine and tri-n-propylamine are preferably used for this . This synthesis method takes place in two steps. First, aqueous formaldehyde solution is reacted with isobutyraldehyde in a stirred tank reactor by heating in an exothermic reaction to form 3-hydroxy-2,2-dimethylpropanal. This is then catalytically hydrogenated to neopentyl glycol at temperatures of 110 to 140 ° C. and pressures of 80 to 150 bar on nickel contacts . The hydrogenation takes place in the homogeneous liquid phase with an aliphatic alcohol (e.g. isobutanol or methanol ) in a tubular reactor.
As already described above, tertiary alkylamines such as trimethylamine , triethylamine , tri-n-propylamine , methyldiethylamine, methyldiisopropylamine or tributylamine are predominantly used in this reaction . These have the advantage that they are very volatile and can be removed from the reaction mixture by distillation . In addition to the organic bases , however, inorganic bases such as alkali hydroxides ( sodium or potassium hydroxide ) and alkaline earth hydroxides can also be used.
properties
Physical Properties
Neopentyl glycol has a density of 1.06 g / cm³ at 128 ° C and a vapor pressure of 0.03 hPa at 20 ° C. 2,2-Dimethylpropane-1,3-diol is hygroscopic at relative humidities of more than 50% and sublimates easily even below its melting point .
Chemical properties
Neopentyl glycol is a colorless, sweet-smelling solid from the organochemical group of alkanediols , i.e. a dihydric alcohol . It is well soluble in water and very soluble in diethyl ether and ethanol . In chemical reactions, neopentyl glycol shows the typical properties of an alcohol, such as ester , ether and carbamate formation. Because of its terminal hydroxyl groups , it can form six-membered, cyclic derivatives with carbonyl compounds or in carbonates , phosphites , sulfites and borates .
use
Neopentyl glycol is mainly used as a component in polyester resins for paints , unsaturated polyester resins, lubricants and plasticizers . It is also used in the production of alkyd resins , synthetic fibers , as well as polyurethanes and additives.
In the future, neopentyl glycol could also be used as a coolant for processors or refrigerators due to its barocaloric properties (temperature change with volume change)
safety instructions
Neopentyl glycol can severely irritate or burn mucous membranes and eyes (depending on the concentration). Chronic hazards have not yet been identified, nor have reproductive toxicity, mutagenicity or carcinogenicity.
Individual evidence
- ↑ entry to neopentyl GLYCOL in CosIng database of the European Commission, accessed on 17 April 2020th
- ↑ a b c d e f g h i j Entry on 2,2-dimethyl-1,3-propanediol in the GESTIS substance database of the IFA , accessed on December 8, 2018(JavaScript required) .
- ↑ a b c d e Entry on 2,2-dimethylpropane-1,3-diol. In: Römpp Online . Georg Thieme Verlag, accessed December 8, 2018.
- ↑ Kurt Schalapski, Dr. Tonia Kretz, Dr. Thorsten Kreickmann, Dr. Peter Heymanns, Dr. Rainer Lukas, Dr. Rolf-Peter Schulz: Process for the production of neopentyl glycol. In: http://www.freepatentsonline.com . Oxea Deutschland GmbH, July 1, 2010, accessed December 8, 2018 .
- ^ Neopentyl glycol (NPG). In: BASF product search. BASF SE, accessed December 8, 2018 .
- ↑ Zhidong Zhang, Kenji Nakajima, Osami Sakata, Shangchao Lin, Weijun Ren: Colossal barocaloric effects in plastic crystals . In: Nature . tape 567 , no. 7749 , March 2019, ISSN 1476-4687 , p. 506-510 , doi : 10.1038 / s41586-019-1042-5 ( nature.com [accessed April 4, 2019]).