Phosphonates
Phosphonates (outdated: phosphites) are salts and organic compounds of the phosphonic acid H 3 PO 3 (outdated: phosphoric acid). There are primary (M'H 2 PO 3 ) and secondary (M ' 2 HPO 3 ) phosphonates as salts. (M ': monovalent metal)
The organic compounds of this group of substances ( phosphorous acid esters ) have the general structure R-PO (OH) 2 (R = alkyl radical or aryl radical ) and differ from the esters of phosphoric acid in that phosphorus is directly bonded to carbon (CP bond ). In contrast, in the case of phosphates (analogous to sulfates and sulfones ) there are COP bonds that are much easier to hydrolyze compared to CP bonds . In compounds of this type, the properties of a salt (or an acid) are linked to the properties of organic compounds. There are many compounds of this type that are soluble in water.
Some technically important phosphonates carry amino group (s) of the type NR 2 - (CH 2 ) x -PO (OH) 2 (R = alkyl or H). Some of these aminophosphonates have structural similarities with complexing agents such as EDTA , NTA or DTPA and have a similar function. They can coat cations such as Ca 2+ in the solution and change the chemical behavior of the cation. In the case of calcium, the property of forming water hardness disappears . However, other cations can also be coated in order to more or less weaken their chemical reactivity.
Natural occurrence
In 1959, the first natural phosphonic acid was identified with 2-aminoethylphosphonic acid. It occurs in plants and many animals, especially in membranes. Phosphonates are widespread in many different organisms, e.g. B. in prokaryotes, eubacteria, fungi, molluscs and insects. The biological role of phosphonates has not yet been finally clarified. To date, no natural bisphosphonates or other polyphosphonates have been discovered.
Technical applications
An important industrial use of phosphonates is in cooling water systems, desalination plants and in oil production, where they prevent the precipitation of salts. In the paper and textile industry, they are used as a stabilizer for peroxide bleaching , where they complex metals that would otherwise inactivate the peroxide.
In detergents, they are used as a combination of complexing agents, to prevent precipitation and as a bleach stabilizer. They can have properties similar to EDTA and can serve as a substitute for pentasodium triphosphate . They are complexing agents and serve primarily as water softeners . As a builder , they can support the ion exchanger or the softener zeolite A as a carrier. They can act as corrosion inhibitors or serve as a stabilizer for peroxides (e.g. in bleaches ).
In 1998, about 56,000 tons of phosphonates were used worldwide - 40,000 tons in the US, 15,000 tons in Europe, and less than 800 tons in Japan.
Examples
| Abbreviation | Chemical names | CAS number | structure |
|---|---|---|---|
| HEDP | 1-hydroxyethane (1,1-diphosphonic acid) | 2809-21-4 |

|
|
ATMP NTMP |
Aminotrimethylenephosphonic acid or amino-tris (methylenephosphonic acid), the same as NTMP , nitrilotris (methylenephosphonic acid) |
6419-19-8 |

|
| DTPMP | Diethylenetriaminepenta (methylenephosphonic acid) or diethylenetriamine-penta (methylenephosphonic acid) |
As sodium salt: 22042-96-2 |

|
| EDTMP | Ethylenediamine tetra (methylenephosphonic acid ) or also ethylenediamine tetra (methylenephosphonic acid) |
As Na salt: 15142-96-8 |

|
| PBTC | Phosphonobutane-tricarboxylic acid 2-phosphonobutane-1,2,4-tricarboxylic acid 3-carboxy-3-phosphonoadipic acid |
37971-36-1 |
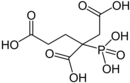
|
Herbicides
One of the best known and most economically important phosphonates is glyphosate (trade name: Roundup ), a herbicide of Monsanto , which contains a phosphonate, a carboxylate and an amino group.
toxicology
The toxicity of the phosphonates mentioned in the examples is low. The LC50 values for fish are between 0.1 and 1 mM. They are also practically not bioaccumulated. These phosphonates are only very poorly absorbed in the gastrointestinal tract and most of the amount absorbed is excreted through the kidneys. The human toxicity of these phosphonates is also very low. Phosphonates are similar to phosphates, but have a CP bond instead of a COP bond. This similarity often means that phosphonic acid esters are inhibitors of many enzymes.
Biodegradation
In nature, bacteria play an important role in the breakdown of phosphonates. Bacteria that can cleave the CP bond can survive with phosphonates as the sole P source. Aminophosphonates can also be used as a nitrogen source by some organisms. The polyphosphonates used in industry differ from the natural phosphonates in that they are much larger, carry a strong negative charge, and are complexed with metals. Degradation tests with sewage sludge have shown that HEDP and NTMP are not degraded. No degradation of HEDP, NTMP, EDTMP and DTPMP was found in standard tests either. Bacteria could be isolated from different environmental compartments (soil, water, sewage sludge, compost), which are able to break down HEDP under P deficiency. In general, bacteria only break down phosphonates with a functional group.
Environmental behavior
The phosphonates mentioned in the examples have some properties which distinguish them from other complexing agents and which determine their environmental behavior. Phosphonates react extremely strongly with surfaces, which leads to a strong elimination in technical and natural systems through adsorption, although they are not biodegradable. Therefore phosphonates hardly lead to metal mobilization in the environment. The photochemical degradation of Fe (III) phosphonates is rapid. Aminopolyphosphonates are rapidly oxidized in the presence of manganese (II) and oxygen; the stable products of this reaction have been identified in sewage treatment plants. The lack of data on environmental behavior or concentrations of phosphonates in the environment comes from analytical problems with trace analysis. In natural waters, phosphonates mainly occur as Ca and Mg complexes.
In the medicine
Phosphonates are used in medicine, especially for the treatment of bone diseases and calcium metabolism disorders. In medicine, bisphosphonates are compounds of phosphonic acid with the general constitutional formula ((O 3 P) 2 -CR 1 R 2 ) 4− . They are analogues to diphosphates (pyrophosphates) (O 3 P-O-PO 3 ) 4− and intervene in calcium homeostasis . They are therefore used, among other things, to treat osteoporosis and bone metastases .
synthesis
Mono- and dialkyl phosphonates can be prepared via the Michaelis-Arbuzov reaction . Halogenated phosphonates can be prepared by the Michaelis-Becker reaction . Silyl phosphonates can be generated by a Kinnear-Perren reaction .
literature
- Philippe Savignac, Bogdan Iorga: Modern Phosphonate Chemistry . CRC Press, 2003, ISBN 0-203-50367-8 .
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 794-795.
- ↑ a b c d e f g h i Eugenia Valsami-Jones: Phosphorus in Environmental Technology: Principles and Applications . IWA Publishing, 2004, ISBN 978-1-84339-001-5 , pp. 149 ( limited preview in Google Book search).
- ↑ Timothy Carl Radsick: The Use of Functionalized Monoalkyl Phosphates and Phosphonates in the ... ProQuest, 2007, ISBN 978-0-549-39821-9 , pp. 36 ( limited preview in Google Book search).
- ^ Abraham Clearfield, Konstantinos D. Demadis: Metal Phosphonate Chemistry: From Synthesis to Applications . Royal Society of Chemistry, 2011, ISBN 978-1-84973-356-4 , pp. 172 ( limited preview in Google Book search).
- ↑ Robert Engel: Handbook of organophosphorus Chemistry . CRC Press, 1992, ISBN 0-8247-8733-1 , pp. 280 ( limited preview in Google Book search).
- ^ Philippe Savignac, Bogdan Iorga: Modern Phosphonate Chemistry . CRC Press, 2003, ISBN 0-203-50367-8 , pp. 521 ( limited preview in Google Book search).


