Alkyd resins

Alkyd resins are synthetic hydrophobic polymers that are formed by the condensation of polyhydric alcohols with polyprotonic acids with the addition of oils or fatty acids (to modify the properties of the resin). Chemically, they belong to the polyesters and are related to the polyester resins , although no long-chain fatty acids are used in their production.
As a polyhydric alcohol, v. a. Glycerin , mainly phthalic acid (or its anhydride ) used as a polyprotonic acid ; the structure shown, however, shows a polyester made from glycerine with isophthalic acid with the incorporation of linoleic acid .
The term alkyd was introduced by RH Kienle in 1927 and is made up of alcohol and acid , the English term for acid . To distinguish it from the oil paints based on hardening oils and natural resins that have been used to date , alkyd resin paints are also referred to as synthetic resin paints .
Alkyd resins are differentiated according to their oil content
- <40%: short oil alkyd resins
- 40 to 60%: medium-oil alkyd resins
- > 60%: long oil alkyd resins
as well as according to the drying behavior
- air drying alkyd resins
- oven-drying alkyd resins
- non-drying alkyd resins
Air-drying alkyd resins polymerize under the influence of oxygen via the double bonds of the unsaturated fatty acid residues. The drying behavior depends on both the proportion and the type of fatty acids built into the polymer. Polyunsaturated fatty acids , such as the linoleic acid shown in the formula or especially the α-linolenic acid , are particularly reactive . Monounsaturated fatty acids, such as oleic acid, harden very slowly. Catalysts (so-called siccatives ) are usually added to accelerate drying ; these are mostly metal soaps made of cobalt or manganese .
Oven-drying alkyd resins contain small amounts of polyunsaturated fatty acids, so that they do not cure sufficiently at room temperature; They are used as components in oven-drying paints (stoving paints), mostly in combination with other synthetic resins.
Non-drying alkyd resins are used as polymeric plasticizers , e.g. B. used in nitrocellulose paints ( nitro combination paints ).
Modified alkyd resins
Alkyd resins are often modified to improve their properties, e.g. B. with isocyanates ( urethane alkyds ), with styrene ( styrenated alkyd resins ) or with acrylic acid or. Methacrylic acid esters ( acrylated alkyd resins ). The modification of alkyd resins is presented in detail below.
| Type of modification | Resulting property improvement |
|---|---|
| Polystyrene / polyacrylic acid ester |
|
| Resole (PF) |
|
| Epoxy resins |
|
| Silicones |
|
| Isocyanates |
|
| Polyamides |
|
Styrene / acrylic ester modification
To modify the alkyd resins with styrene , polyunsaturated fatty acids are first required in the chemical structure of the alkyd resins. These react in the presence of peroxides as initiators (e.g. di-tert-butyl peroxide ) at approx. 140–170 ° C with free radical polymerization . This sometimes creates polymer bridges between the fatty acid molecules and branches. The resulting higher molecular weights lead to better resistances. The high TG of the copolymer also increases the resistance and also ensures faster drying. The fact that the double bonds of the fatty acids react with the copolymer reduces the tendency to yellowing, but through-drying in paints is also worsened.
Modification with epoxy resins
This requires alkyd resins which still have a very high acid number . The reaction of the carboxyl group with epoxides results in epoxy resin-modified alkyd resins. The epoxy resin has the function of an oligomeric alcohol derivative. Above all, the modification achieves better adhesion to metal and increased corrosion protection . The following is a simplified illustration of the reaction. The basic structure of the epoxy resin R can be composed of bisphenol A and epichlorohydrin . Polyunsaturated C18 fatty acids can be used as R2 in the alkyd resin skeleton.
Modification with silicones
Here oligomeric siloxanes are used, which are built into the basic structure of the alkyd resins. The water resistance increases due to the hydrophobic properties of the siloxanes. In addition, the weather and chemical resistance is also improved. Since silicones have a high temperature resistance, this is also improved.
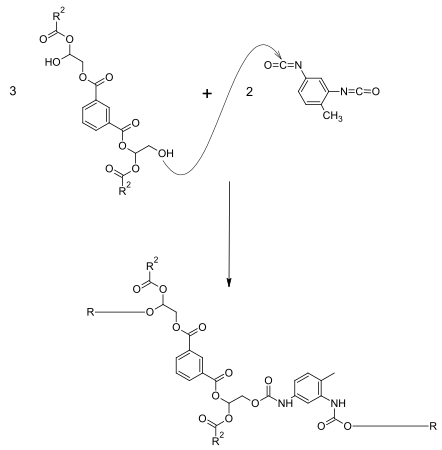
Modification with isocyanates
If parts of phthalic anhydride are replaced by diisocyanates (e.g. TDI ), a urethane modification is obtained. For this purpose, alkyd resins with a low molar mass and a high OH content are first produced. This is followed by the reaction with a diisocyanate. Depending on the content of urethane groups, a high degree of hardness with simultaneous elasticity can be achieved. Because of the lower proportion of ester groups , the chemical resistance, especially the alkali resistance, is improved. The weather resistance depends on whether an aromatic or aliphatic isocyanate is used. A reaction example for producing a urethane-modified alkyd resin is shown below. The isocyanate (here TDI) reacts with the OH groups of the alkyd resin. In order to obtain OH-functional products, the OH groups must be present in excess.
If phthalic anhydride is completely replaced by the diisocyanate, so-called urethane oils are obtained.
Modification with polyamides
Alkyd resins often show a slight, time-independent, shear thinning . This structural viscosity comes from the interactions between the dissolved macromolecules and is increased by a higher concentration of the solution. A thixotropic behavior is achieved if one incorporates segments that have a strong tendency to associate with one another , but are insoluble in the dissolved solvent. Strong networks are formed which are destroyed by shear and only rebuild after a certain time. The thixotropy decreases again with the addition of polar solvents, since the interactions between the associative groups are interrupted.
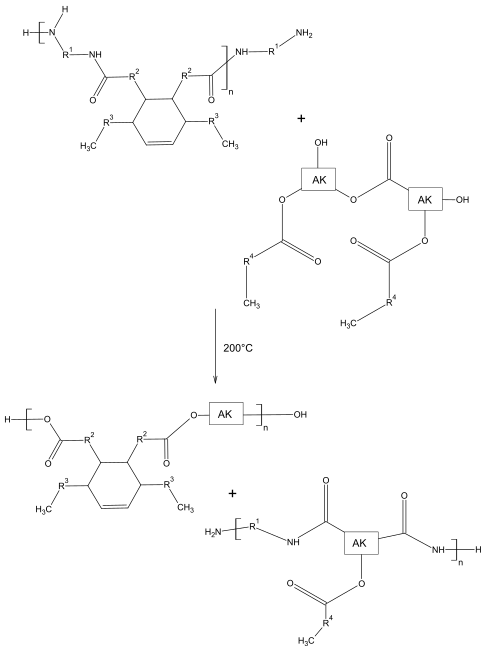
Thixotropic alkyd resins are formed by reacting with polyamides . Polyamides made from polyamines and dimer fatty acids are particularly suitable . In contrast to many polyamides, these are not crystalline and only partially soluble in non-polar solvents.
At approx. 200 ° C, transamidation occurs together with an alkyd resin. The amide groups newly formed on the alkyd resin ensure the formation of the networks. The reaction is shown schematically below. Possible residues are C18 fatty acids. The product is able to form networks via hydrogen bonds .
The boiling time is of decisive importance for the later thixotropy of the binder. If this is too low, the conversion is still too low and no thixotropy can be developed. If the boiling time is too long, you would have a completely tolerable solution, also without thixotropic behavior.
The clear point can serve as an indication of the optimal boiling time. The solution becomes cloudy due to the incompatibility between polyamide and alkyd resin. This cloudiness disappears over time and the reaction can then be stopped. In addition, several samples can be taken over the boiling time and the rheological behavior can be determined. The time until maximum thixotropy can then serve as a guideline for the manufacturing process.
use
Alkyd resins are mainly used as:
- Alkyd resin paint
- Leveling compounds
- Additives (auxiliary materials)
Individual evidence
- ↑ Dieter Stoye, Werner Freitag: Lackharze . Carl Hanser Verlag, 1996, ISBN 3-446-17475-3 .
- ^ A. Spyros: Characterization of Unsaturated Polyester and Alkyd Resins Using One- and Two-Dimensional NMR Spectroscopy . In: Journal of Applied Polymer Science 88, 2003, pp. 1881-1888. ISSN 0021-8995 .
- ^ T. Nagata: Cooking Schedule of Alkyd Resin Preparation. Part II. Effect of Cooking Schedule on Molecular Weight Distribution of Alkyd Resin . In: Journal of Applied Polymer Science 13, 1969, pp. 2601-2619. ISSN 0021-8995 .
- ↑ a b c d Entry on alkyd resins. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ a b Kittel, textbook of paints and coatings, 2nd edition, volume 2: Binder for solvent-based and solvent-free systems, Ed. Walter Krauß, s. Hirzel Verlag 1998, ISBN 3-7776-0886-6 .
- ^ Goldschmidt and Streitberger: Lackiertechnik , 2002.
- ↑ Poth, Ulrich-: Polyester and alkyd resins: Fundamentals and applications . 2., revised. Vincentz Network, Hannover 2014, ISBN 978-3-86630-662-2 , p. 197-204 .