Isophthalic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
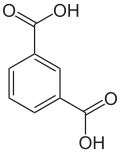
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isophthalic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 6 O 4 | |||||||||||||||
| Brief description |
colorless and almost odorless crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 166.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.54 g cm −3 |
|||||||||||||||
| Melting point |
346 ° C |
|||||||||||||||
| Sublimation point |
348 ° C |
|||||||||||||||
| Vapor pressure |
610 Pa (250 ° C) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
sparingly soluble in water: 0.12 g l −1 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
2 mg m −3 (inhalable aerosol fraction) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Isophthalic acid (also meta - phthalic acid ) is a chemical and belongs to the group of aromatic dicarboxylic acids ( benzene dicarboxylic acids ).
Physical Properties
The ignition temperature is 700 ° C.
Manufacturing
Isophthalic acid is produced by the oxidation of meta- xylene with atmospheric oxygen.
use
Isophthalic acid is used as a starting material for the production of aramids , polyesters , synthetic resins for high-temperature-resistant electrical insulating varnishes and oil-free alkyd resins .
Individual evidence
- ↑ a b c d e f g Entry on isophthalic acid in the GESTIS substance database of the IFA , accessed on December 21, 2019(JavaScript required) .
- ↑ a b Data sheet isophthalic acid (PDF) from Merck , accessed on August 14, 2007.
- ↑ a b c CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on isophthalic acid. In: Römpp Online . Georg Thieme Verlag, accessed on July 17, 2014.