Toluene-2,4-diisocyanate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Toluene-2,4-diisocyanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 6 N 2 O 2 | |||||||||||||||
| Brief description |
colorless to yellowish, pungent smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 174.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.22 g cm −3 |
|||||||||||||||
| Melting point |
21 ° C |
|||||||||||||||
| boiling point |
251 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Toluene-2,4-diisocyanate (TDI) is one of the most important isocyanates and an important intermediate in the plastics industry. At the end of the 1930s, the compound, along with other isocyanates, proved to be an ideal starting substance for polyaddition reactions and was classified by Otto Bayer and his colleagues in Leverkusen as a promising product for the production of foams ( polyurethanes ).
properties

Toluene-2,4-diisocyanate is a very toxic liquid. TDI is readily soluble in organic solvents (for example in benzene or in toluene ); it reacts with water with relatively slow decomposition (formation of polymeric ureas and carbon dioxide ). Therefore, organic solvents must be anhydrous if they are to be used for synthesis with TDI.
The compound forms flammable vapor-air mixtures at higher temperatures. Their flash point is 127 ° C, the explosion range is between 0.9% by volume (64 g / m 3 ) as the lower explosion limit (LEL) and 9.5% by volume (685 g / m 3 ) as the upper explosion limit (OEG). The ignition temperature is 620 ° C. The substance therefore falls into temperature class T1.
The market is dominated by three different isomer mixtures or products: the largest share by far is made up of 80% toluene-2,4-diisocyanate and 20% toluene-2,6-diisocyanate under trade names such as "Desmodur T 80" ( Covestro AG ) or “Lupranat T 80” ( BASF AG ), also T 65 made from 65% toluene-2,4-diisocyanate and 35% toluene-2,6-diisocyanate. T 100, on the other hand, consists of almost 100% toluene-2,4-diisocyanate.
Worldwide annual capacities are around 1.5 million tons (as of 2005) and TDI is the most widely produced isocyanate alongside MDI . Today it is produced in large plants around 100,000 tons and more annually, e.g. B. in Dormagen , Brunsbüttel , Schwarzheide (D), in Baytown (USA), in Japan and since 2006 in Caojing (China). In November 2016, the world's largest production facility operated by Sadara Chemical Company , a joint venture between Dow Chemical and Saudi Aramco , went into operation in Al-Jubail (Saudi Arabia) . In November 2015, BASF put a TDI plant with a capacity of 300,000 metric tons a year into operation at the Ludwigshafen site (costs approx. 1 billion euros).
Smaller plants in Leverkusen , Tarragona (E), Antwerp (B) and New Martinsville (USA) were closed at the end of the 20th and beginning of the 21st century as part of global consolidation measures.
Manufacturing
TDI can be produced in different ways:
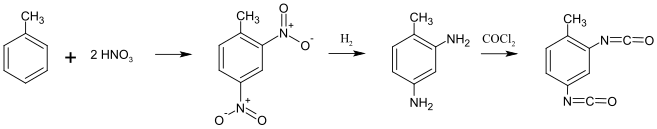
The route via the nitration of toluene and the subsequent catalytic hydrogenation with hydrogen to 2,4-diaminotoluene (2,4-toluene diamine, TDA), which is finally phosgenated, is a technically mature process with high yields .
A disadvantage of this process is that the highly toxic phosgene has to be used as a reactant and that most chemical plants producing this way operate with a slight overpressure. However, this base phosgenation is still the most economical production process and the most widely used worldwide. It has been continuously optimized since the first large-scale system was commissioned in Leverkusen in 1953. In order to keep the risk of a phosgene outbreak or exposure of this gas in the environment as low as possible, the safety precautions of such a chemical plant are very strictly regulated and redundant . In Germany it is subject to the Hazardous Incident Ordinance . The basic principle is to keep the amount of phosgene located during the process in circulation as low as possible (no Phosgenlagertanks etc., see Bhopalunglück ). In the event of a malfunction, the system must switch off automatically and the phosgene that escapes must be destroyed as quickly as possible by ammonia vapor. There are special safety devices in industry for this purpose. Through phosgenation in the gas phase, i.e. without a solvent as a carrier liquid, a chemical plant can be operated without pressure, i.e. with a slight negative pressure . This makes gas leaks extremely difficult from a purely physical point of view.
Alternatively, TDI can be produced by the oxydehydrogenation of formamides . This eliminates the need to handle phosgene. However, this is not yet a mature process.
Base phosgenation of TDA
The process runs in several steps in a continuously operating chemical plant. These steps are in detail:
- Phosgene production
- Phosgene absorption
- Phosgenation
- Dephosgenation
- Solvent distillation
- Residue separation
- Isocyanate purification
The carrier of the starting materials phosgene and toluene diamine (slightly alkaline, therefore base ) and the reaction products is the solvent ortho-dichlorobenzene (ODB or ODCB). The starting materials are both mixed with them before the reaction; the product TDI must then be separated from it by distillation processes. The recovered ODB is reused in a circular process.
For the production of phosgene, the gases chlorine and carbon monoxide (CO) are first mixed, whereby there is no reaction due to the high activation energy (95 kJ / mol) . By passing the mixed gas over the activated carbon catalyst , this can be reduced to about 30 kJ / mol. This means that the reaction starts at around 40–50 ° C. With complete conversion, 106.7 kJ / mol of energy are released. The reaction is therefore exothermic , the heat generated in the reactor must be transported away by a cooling device.
Of these three substances, chlorine has the greatest affinity for activated carbon and prefers to adsorb . At the same time, it is ensured that phosgene formed and adsorbed is immediately desorbed by the incoming chlorine . In order to be able to completely convert the chlorine, a small excess of carbon monoxide is guaranteed. The outlet temperature of the resulting gas mixture must be well below 100 ° C so that no dissociation is noticeable. As the temperature rises, phosgene begins to break down again into the starting materials (at 200 ° C approx. 5% phosgene has dissociated, at 800 ° C practically only CO and chlorine are present).
The phosgene is now dissolved in cold ODB. The solubility is high, so that mass fractions of 60% COCl 2 and more can be achieved. The toluenediamine (TDA), which was previously melted at approx. 140 ° C., is diluted in a hot ODB.
The phosgenation reactions are composed of a small number of main reactions and a myriad of side reactions. They can be summarized in two sub-process steps, cold and hot phosgenation . Before starting it is important to use a large excess of phosgene. This should be at least 300%, but if possible not higher than 400%. The decisive factor here is the proportion of 2,4-TDA in the isomer mixture. The greater this is, the higher the phosgene excess must be, since the sterically favorable 4-position of the second amino group can be reached more easily by phosgene molecules than the 6-amino group of 2,6-TDA, which is laterally shielded by the methyl group . Too high a phosgene excess would lead to an undesirably rapid phosgenation.
In simplified terms, the first exothermic main reactions of cold phosgenation result in five solid intermediate products that form a suspension in ODB:
Carbamic acid chloride, bis-carbamic acid chloride, amine hydrochloride, bis-amine hydrochloride and compounds with both chemical groups
These intermediate products now have to be converted further by adding a lot of heat . Hydrogen chloride (HCl) is split off endothermically from the carbamic acid chlorides through the action of heat , resulting in TDI. Furthermore, phosgene also converts the amine hydrochloride into TDI with repeated exclusion of HCl. The solubility of hydrogen chloride in ODB is hardly present, so that outgassing of the reaction hydrogen chloride occurs immediately.
During the hot phosgenation, the formation of some undesirable and irreversible, residue-forming secondary compounds (ureas, biurets, isocyanurates, etc.) is unavoidable, but must be kept to a minimum. The course for a high yield (95% and more) must already be set in the cold phosgenation through optimal mixing, flow and temperature conditions. The construction of the hot phosgenation reactors is extremely important. So that there is no unwanted post-chlorination by the hydrogen chloride or overphosgenation, the residence times of the molecules must be tightened. Ideally, all molecules should have approximately the same residence time. For this purpose, the tower-like, cylindrical reactors are slim and tall, backflows are prevented by many perforated floors or at least limited to the space between two floors (chambering). Most of the heat required for the endothermic reactions should be supplied immediately upon entry into the reactor in order to achieve the fastest possible conversion to TDI. The apparatus material of these hot phosgenation towers must have a high nickel content due to the highly corrosive HCl vapor (e.g. Inconel 600).
At the outlet of the hot phosgenation reactors, the liquid raw material (approx. 15% TDI, 80% ODB, 5% phosgene and residue products from side reactions) is inevitably separated from the hot process gas (phosgene excess, HCl). The process gases go back to the phosgene absorption where the phosgene on the one hand is returned to the phosgene solution and on the other hand the hydrogen chloride is discharged. This is absorbed in a separate plant in approx. 18% hydrochloric acid in an azeotropic process . The resulting 30% hydrochloric acid is very pure and can be used for several purposes.
In parallel, the raw material is dephosgenated in a first distillation step, in which a large part of the still dissolved phosgene is separated off using heat. This is followed by the distillative separation of the ODB, the residue and the purification of the TDI from undesired secondary components such. B. hydrolyzable chlorine compounds or carbon tetrachloride . This takes place in the best case in series-connected distillation columns , but may be in a single, suitably designed column having for example. Bubble cap trays are performed. Due to the high boiling point of TDI, these process steps are carried out as vacuum distillations .
use
In the chemical industry, TDI is an important intermediate product for the production of adhesives , foams for mattresses and upholstery, polyurethanes (e.g. for shoe soles), elastomers , coatings and high-quality paints for use in the automotive industry, for painting aircraft or railcars as well as for the production of lubricants.
safety instructions
The vapors are very irritating to the eyes and respiratory tract. After long-term inhalation of the vapors, pulmonary edema is possible, which can occur with a delay. The occupational exposure limit is very low at 0.005 ppm , as long-term effects on the lungs can lead to sensitization of the respiratory tract and even asthmatic diseases.
Web links
Individual evidence
- ↑ a b c d e f g h i j k Entry on toluene-2,4-diisocyanate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d Datasheet Toluene 2,4-diisocyanate from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Entry on 4-methyl-m-phenylene diisocyanate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c E. Brandes, W. Möller: Safety characteristics - Volume 1: Flammable liquids and gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.