Dichlorobenzenes
In chemistry, dichlorobenzenes form a group of substances consisting of a benzene ring with two chlorine atoms (–Cl) as substituents . Their different arrangements ( ortho , meta or para ) result in three constitutional isomers with the empirical formula C 6 H 4 Cl 2 .
Representative
| Dichlorobenzenes | ||||||||||
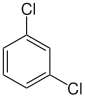
| Surname | 1,2-dichlorobenzene | 1,3-dichlorobenzene | 1,4-dichlorobenzene | |||||||
| other names | o -dichlorobenzene | m -dichlorobenzene | p -dichlorobenzene | |||||||
| Structural formula |

|
|

|
|||||||
| CAS number | 95-50-1 | 541-73-1 | 106-46-7 | |||||||
| 25321-22-6 (mixture of isomers) | ||||||||||
| PubChem | 7239 | 10943 | 4685 | |||||||
| Molecular formula | C 6 H 4 Cl 2 | |||||||||
| Molar mass | 147.00 g mol −1 | |||||||||
| Physical state | liquid | firmly | ||||||||
| Brief description | colorless liquid with an unpleasant odor |
colorless liquid with a characteristic aromatic odor |
white crystalline solid with a camphor-like odor |
|||||||
| density | 1.32 g cm −3 (20 ° C) | 1.29 g cm −3 (20 ° C) | 1.248 g cm −3 (20 ° C) | |||||||
| Melting point | −18 ° C | −22 ° C | 53 ° C | |||||||
| boiling point | 179 ° C | 173 ° C | 174 ° C | |||||||
| Vapor pressure (20 ° C) | 1.33 hPa | 2.4 hPa | ||||||||
| solubility | 0.08 g l −1 (20 ° C) | 0.11 g l −1 (20 ° C) | 0.049 g l −1 (20 ° C) | |||||||
| practically insoluble in water, soluble in organic solvents | ||||||||||
| Flash point | 70 ° C | 63 ° C | 66 ° C | |||||||
| Lower explosion limit (LEL) | 1.7 vol% | 1.7 vol% | ||||||||
| 103 g m 3 | ||||||||||
| Upper explosion limit (UEL) | 12 vol% | 5.9 vol% | ||||||||
| 735 g m 3 | ||||||||||
| Maximum explosion pressure | 6.9 bar | |||||||||
| Ignition temperature | 640 ° C | > 500 ° C | 640 ° C | |||||||
| Temperature class | T1 | |||||||||
|
GHS labeling |
|
|
|
|||||||
| H and P phrases |
302 + 332-315-319 317-335-410 |
302-411 | 319-351-410 | |||||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||||
|
261-280-301 + 312 + 330 305 + 351 + 338 |
273 |
273-280-305 + 351 + 338 308 + 313 |
||||||||
| MAK | Switzerland: 10 ml m −3 or 61 mg m −3 | Switzerland: 2 ml m −3 or 12 mg m −3 | Switzerland: 2 ml m −3 or 12 mg m −3 | |||||||
presentation
If benzene is used directly with chlorine in the presence of a Lewis acid such as. B. iron (III) chloride or aluminum chloride reacted, the main product is chlorobenzene in 80-90% yield. The o - and p -dichlorobenzenes are only obtained as by-products in small amounts in this reaction.
If the amount of chlorinating agent used is increased significantly, larger amounts of p -dichlorobenzene can be obtained in addition to o -dichlorobenzene and a little m -dichlorobenzene. Furthermore, chlorobenzene and the more highly chlorinated benzenes (e.g. trichlorobenzenes ) are also produced.
properties
o -Dichlorobenzene and m -Dichlorobenzene are colorless oily liquids with a pungent odor; p -Dichlorobenzene (also called paradichlorobenzene (PDCB)) is a solid with a strong odor. The substance is found in the air and in water worldwide and is difficult to biodegrade.
Dichlorobenzenes are practically insoluble in water, but soluble in organic solvents. The boiling points of the three isomers are relatively close to one another, while their melting points differ more clearly. The p -dichlorobenzene, which has the highest symmetry, has the highest melting point.
use
Dichlorobenzenes are increasingly used as starting materials and intermediates for pharmaceuticals, pesticides, dyes and pesticides. They serve as solvents for paints, rubber, waxes, resins and disinfectants.
Most of the o- dichlorobenzene produced is processed further to 3,4-dinitrochlorobenzene by nitration . The substance is also used as an inert solvent in the production of isocyanates . The m -dichlorobenzene is used exclusively as a chemical intermediate.
For p- dichlorobenzene (PDCB), which occurs as a by-product, some very controversial areas of application have been found in the past. In many toilet stones , the fabric has now been replaced by alternative fabrics. In stones for urinals in gastronomy it is still used today because it has a strong, raspberry-like smell. However, the substance has no germicidal effect. PDCB is also still used in mothballs and pesticides.
Hazard assessment
p -Dichlorobenzene is poorly degradable in the environment . It is toxic to aquatic organisms (WGK 2) and can therefore have long-term harmful effects. PDCB has a harmful effect in the animal organism by primarily attacking the liver, kidneys and lungs. It is also irritating to the skin and eyes. In more recent studies, paradichlorobenzene has been shown to be carcinogenic (carcinogenic / carcinogenic).
In 2012, 1,2-dichlorobenzene was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of 1,2-dichlorobenzene were concerns regarding high (aggregated) tonnage and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances. The re-evaluation took place from 2013 and was carried out by Hungary .
Web links
- Process for the separation of m - and p -dichlorobenzene
- Production of dichloroaromatics in the presence of Lewis acids and HCl
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on o-dichlorobenzene in the GESTIS substance database of the IFA , accessed on March 4, 2020(JavaScript required) .
- ↑ a b c d e f g h i Entry on m-dichlorobenzene in the GESTIS substance database of the IFA , accessed on March 4, 2020(JavaScript required) .
- ↑ a b c d e f g h i j k record with p-dichlorobenzene in the GESTIS database of IFA , accessed on March 4, 2020(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1,2-dichlorobenzene ), accessed on March 4, 2020.
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limit values - current MAK and BAT values (search for 1,3-dichlorobenzene ), accessed on March 4, 2020.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1,4-dichlorobenzene ), accessed on March 4, 2020.
- ↑ S. Braverman, M. Cherkinsky, ML Birsa: Four Carbon-heteroatom bonds . In: JG Knight (Ed.): Science of Synthesis: Houben-Weyl Methods of Molecular Transformations . tape 18 . Georg Thieme Verlag, Stuttgart 2014, ISBN 978-3-13-178091-1 , p. 63 ( limited preview in Google Book search).
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 4: M-Pk. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1985, ISBN 3-440-04514-5 , p. 2679.
- ↑ a b Umweltlexikon: P. (PDCB) occurs as a by-product in the production of monochlorobenzene (solvent), for 1980 the production volume was estimated at at least 80,000 t. (from January 17, 2001)
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1,2-dichlorobenzene , accessed on May 20, 2019.


