Isocyanates
Isocyanates are the esters of the volatile isocyanic acid . The salts of isocyanic acid are identical to the salts of cyanic acid and are therefore referred to as cyanates .
Production of isocyanates
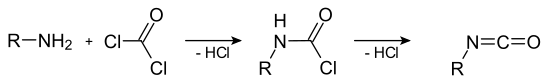
Isocyanates can be produced by reacting the corresponding amines with phosgene as a reactant . The synthesis occurs in two steps over the as intermediate occurring carbamoyl chloride . A hydrogen chloride molecule is split off in each sub-step :
Alternative synthesis routes are, for example, the catalytic carbonylation of nitro compounds or amines or the reaction of primary amines with di- tert-butyl dicarbonate ( Diboc ) in the presence of 4- (dimethylamino) pyridine (DMAP).
Chemical properties
Isocyanates are chemically highly reactive compounds that have the structural element R – N = C = O. The most important reactions of the isocyanate group are addition reactions . They react with alcohols to form urethanes , with amines to form urea derivatives and with water to form carbamic acids ; However, the latter are chemically unstable and decompose directly to the amines , releasing carbon dioxide . They react with carboxylic acids to form amides which, with further isocyanate, form acylureas .
Urethane formation:
Urea derivative formation:
Reaction with water:
Reaction with carboxylic acids (formation of amide or acyl urea):
Isocyanates can dimerize to uretdiones in an equilibrium reaction . The dimerization can be accelerated by basic catalysts such as pyridine or tertiary phosphines ; However, aromatic isocyanates dimerize even without a catalyst.
At higher temperatures, the position of equilibrium is shifted towards the thermally more stable isocyanates.
Trimerization to give the so-called isocyanurates (1,3,5-triazine-2,4,6-triones) is also possible; as catalysts for this are, for. B. sodium formate , potassium acetate , phosphines or tertiary amines are suitable. Isocyanurates produced from diisocyanates are used as a reaction component with a particularly low vapor pressure for polyurethane systems. However, trimerization can also be used to produce polyisocyanurates .
Application and use
When using isocyanates, a distinction must be made between the very volatile monoisocyanates and the much less volatile di- and polyisocyanates.
Methyl isocyanate (CH 3 –N = C = O) is the simplest ester of isocyanic acid. It is a very reactive substance that z. B. is used in the manufacture of pesticides .
Industrial use
If compounds with two isocyanate groups ( diisocyanates ) are reacted with dihydric alcohols ( diols ), polyaddition reactions take place and the industrially diverse polyurethanes are formed . If water (e.g. air humidity) is also present at the same time, the reaction also takes place via the unstable carbamic acids, which break down with the splitting off of carbon dioxide. The resulting gas is responsible for foam formation in polyurethane foams . The amine formed at the same time can, in turn, as shown above, react with a further isocyanate to form a urea compound. When polyisocyanates are cured with water, polyureas are formed. In this way, one-component products (paints, adhesives, foams) can also be formulated, which draw the water required for curing as water vapor from the ambient air. Thin layers cure without foaming, provided the base material has sufficient permeability for carbon dioxide. Due to the reactivity of the isocyanates, the polyurethane coatings adhere very well to most materials. This is why polymer isocyanates are also used as adhesion promoters or primers for other coatings.
The main application of diisocyanates and blocked isocyanates is the synthesis of polyurethanes. The most important representatives of diisocyanates are:
- Toluene-2,4-diisocyanate (TDI)
- Diphenylmethane diisocyanate or methylenediphenyl diisocyanate (MDI)
- Hexamethylene diisocyanate (HDI, HMDI)
- Polymeric diphenylmethane diisocyanate (PMDI)
- meta -Tetramethylxylylene diisocyanate (TMXDI)
- Isophorone diisocyanate (IPDI)
- 4,4'-diisocyanatodicyclohexylmethane (H12MDI)
The last two aliphatic diisocyanates and their derivatives as well as hexamethylene diisocyanate are mainly used in high-quality polyurethane paint systems.
Characterization and classification
There are different possibilities to divide (poly) isocyanates into classes or to characterize them. First of all, a characterization of the material composition can be carried out. The application is usually divided into aromatic or aliphatic isocyanates. This chemical structure results in important properties such as reactivity and weather resistance of the systems and mixtures obtained.
Since the isocyanate groups of the (poly) isocyanates are usually reacted stoichiometrically, i.e. molar 1: 1, with reactants, the amount of isocyanate groups must be described. I. d. As a rule, the so-called isocyanate content is specified here, which can refer to the isocyanate as a pure substance or to the partial solution. The isocyanate content describes the percentage by weight of isocyanate groups in the mixture:
As the isocyanate content is only a little characterizing quantity, the so-called equivalent weight can be given as an alternative. The isocyanate equivalent weight (NCO equivalent weight) describes the amount of substance / mixture which contains exactly one mole of isocyanate groups. Knowledge of the mass of an isocyanate group of 42 g per mol is relevant for this.
If the mixing ratio of a (poly) isocyanate with z. B. a polyester-polyol are calculated, its equivalent weight is also necessary. This OH equivalent weight describes the amount of substance which contains one mole of hydroxyl groups.
Medical importance
Isocyanates damage the cell membranes of human cells by reacting with NH 2 and OH groups. Inhalation of isocyanate-containing vapors causes irritation of the skin and mucous membranes . Corneal damage can be caused in the eye . HMDI, in particular, can cause hives , contact eczema or toxic dermatitis on the skin .
Isocyanates, especially MDI and TDI, can cause a type I allergic reaction. The isocyanate asthma that develops as a result is similar to classic allergic asthma , but seems to be subject to slightly different molecular mechanisms. Specific antibodies are rarely detected. A diagnosis is often made using a provocation test . Less frequently, there is damage to the alveolar epithelium in the lungs with the clinical picture of alveolitis ( isocyanate alveolitis ).
Respiratory diseases caused by isocyanates can be recognized as occupational diseases (BK1315). Employees who are regularly exposed to isocyanates must take part in preventive occupational health examinations . In order to avoid inhalation exposure , the low molecular weight representatives are replaced in most applications by low-volatility derivatives.
toxicity
Isocyanic acid, methyl isocyanate and other isocyanates are very toxic.
On December 3, 1984, around 40 tons of methyl isocyanate (" Bhopal accident ") escaped from a defective tank in a pesticide factory owned by the American chemical company Union Carbide Corporation near the city of Bhopal ( India ). The gas cloud killed more than 2,800 people and caused serious injuries such as damage to the eyes and mucous membranes in several hundred thousand other people .
Individual evidence
- ↑ Hans-Joachim Knölker, Tobias Braxmeier, Georg Schlechtingen: A Novel Method for the Synthesis of Isocyanates Under Mild Conditions. In: Angewandte Chemie International Edition in English. 34, 1995, pp. 2497-2500, doi : 10.1002 / anie.199524971 .
- ↑ a b Hans Kittel: Textbook of paints and coatings. In: Walter Krauß (Hrsg.): Volume 2: Binder for solvent-based and solvent-free systems . 2nd Edition. Hirzel, Stuttgart 1998, ISBN 3-7776-0886-6 .
- ↑ Ulrich Poth: Synthetic binders for coating systems. Vincentz Network, Hannover 2016, ISBN 978-3-86630-611-0 .
- ↑ Jürgen Fritze: The medical assessment: legal questions, functional tests, assessments . 7th edition. Steinkopff, Darmstadt 2008, ISBN 3-7985-1563-8 .
- ↑ Claus Kroegel, Neil C. Barns: Bronchial asthma: pathogenetic bases, diagnostics, therapy . 2nd edition, Georg Thieme, Stuttgart / New York 2001, ISBN 3-13-104732-1 .



![{\ mathrm {R {-} NCO + H_ {2} O \ longrightarrow [R {-} NHCOOH]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/acad08599ab8245d881cb05ff8e9108f258fd5f4)
![{\ mathrm {[R {-} NHCOOH] \ longrightarrow R {-} NH_ {2} + CO_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7bd4910a435e2ccc5efac7cfd8634383a2e44516)



![{\ displaystyle {\ text {isocyanate content}} = {\ frac {({\ text {mass}} [{\ text {isocyanate groups}}])} {\ text {total mass}}} {\ text {in}} [ {\ text {percent}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/afa8ba3b4023c9a694ffcc38aa1cf900eaf580cf)
![{\ displaystyle NCO - {\ text {equivalent weight}} = {\ frac {1} {\ left ({\ frac {{\ text {isocyanate content}} [\%]} {100 \ cdot 42 \ mathrm {\ frac { g} {mol}}}} \ right)}} {\ text {in}} [\ mathrm {g / mol (NCO)}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e8ea593a4c9b63b0888483c465edf9734e1de190)
