meta- tetramethylxylylene diisocyanate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of meta- tetramethylxylylene diisocyanate | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | meta- tetramethylxylylene diisocyanate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 16 N 2 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 244.29 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.06 g cm −3 |
||||||||||||||||||
| Melting point |
−10 ° C |
||||||||||||||||||
| boiling point |
106 ° C at 1.2 hPa (1.2 mbar) |
||||||||||||||||||
| Vapor pressure |
0.4 Pa (° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
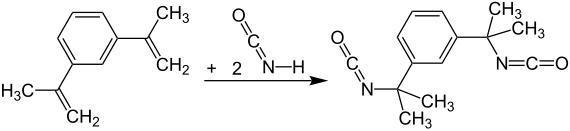
In meta -Tetramethylxylylendiisocyanat ( m -TMXDI) is a chemical compounds from the group of aliphatic or partially aromatic isocyanates or polyisocyanate . TMXDI contains two isocyanate groups which are located on aliphatic radicals in the meta position . The name of all TMXDI types is based on xylene.
Extraction and presentation
Since the name of the TMXDI, as already mentioned, is based on the xylenes (a mixture of three structural isomers ), but it is not synthesized from this, it is possible, e.g. B. specifically to obtain the meta form . An example of production is the addition of isocyanic acid to m- dimethyldivinylstyrene.
properties
The basic structure of the m -TMXDI is based on the structure of the m -Xylene. There are 2 further methyl groups and an isocyanate group on each of the methyl groups of the m- xylene . Due to the aliphatic connection of the isocyanate group, the TMXDI has properties that are similar to those of aliphatic isocyanates such as HDI and IPDI . In contrast to IPDI, both isocyanate groups have the same reactivity
use
In addition to meta-TMXDI, there are other isomers based on the name tetramethylxylylene diisocyanate. The meta form of TMXDI, the name of which is based on m -xylene, is primarily used for technical purposes.
m -TMXDI is used, for example, in aqueous polyurethane dispersions for paints. Here, m -TMXDI can be added to dimethylolpropionic acid and incorporated into a lacquer resin via an excess of isocyanate groups, which ultimately results in water compatibility .
Individual evidence
- ↑ a b c d e f g Entry on 1,3-bis (1-isocyanato-1-methylethyl) benzene in the GESTIS substance database of the IFA , accessed on June 10, 2018 (JavaScript required)
- ↑ Data sheet 1,3-bis (1-isocyanato-1-methylethyl) benzene from Sigma-Aldrich , accessed on June 10, 2018 ( PDF ).
- ↑ Patent US3290350 : Alpha, alpha, alpha ', alpha'-tetramethyl-xylylene diisocyanates and alpha, alpha-dimethylisopropenylbenzyl isocyanates and the preparation thereof from isocyanic acid and olefins. Published December 6, 1966 , inventor: Fred W. Hoover.
- ↑ a b Ulrich Poth: Synthetic binders for coating systems . Vincentz, Hannover 2016, ISBN 3-86630-611-3 , p. 409 .
- ↑ Patent DE19639325 : Process for the production of an aqueous dispersion of a polyacrylate-modified polyurethane-alkyd resin and the use of such a dispersion. Published on March 26, 1998 , inventors: Guido Wilke, Ulrich Poth, Rolf Seidemann, Vijay Kadambande.