Polyurethane dispersion
A polyurethane dispersion , also known internationally as PUD (polyurethane dispersion), is a flowable two-phase system consisting of water and a polymer , i.e. H. a dispersed plastic that belongs to the class of the polyurethanes , and optionally other components.
Polyurethane dispersions are preferably used in the field of coatings as film formers or binders including adhesives, but also for the production of other technical products. Because polyurethane dispersions are viewed as an environmentally friendly alternative to solvent-based binders, they have gained increasing importance in the processing industry.
Properties and characteristics of the dispersion
The general advantages of polyurethane plastics are their flexibility at low temperatures, their outstanding, in some cases selectable mechanical properties, resistance to certain chemicals and, depending on their structure, good to very good weather resistance.
General structure
In order to achieve sufficient properties, however, high molecular weights of the polymers are required. A dispersion has the advantage that, even with very high molar masses, the viscosity is only determined by the particle size of the dispersed plastic and its volume fraction in the dispersion. These systems are therefore superior in coatings to the real solutions of polyurethanes in organic solvents in terms of environmental protection, ie solvent emissions and application efficiency. For this reason, in the last two to three decades, with increasing demands on environmentally friendly processes in the manufacturing industry, polyurethane dispersions have gained a high status.
Polyurethane dispersions are incorrectly referred to as polyurethane emulsions or PU emulsions in the English-speaking world . However, it is neither an emulsion nor the production process of polymer emulsion polymerization . The latter produces a relatively uniform composition with regard to the size of the particles of the plastic produced, while the polyurethane dispersion is produced in a polymer addition process and usually has a fairly broad distribution of different particle sizes.
The polyurethane particles of a stable dispersion are spherical and range in size between about 30 nm and 1000 nm, giving them a milky white (sometimes yellowish) appearance. The size distribution can have both a maximum (monomodal) and two maxima (bimodal). Below 50 nm particle size the dispersion appears increasingly transparent, above 1000 nm the particles tend to settle and the dispersion cannot be stored.

The weight fraction of the plastic ( solids content ) in commercially available polyurethane dispersions is typically 30% to 50%, occasionally even 60%. Dispersions with such a high proportion of solids have advantages in terms of transport and storage, the dry film thicknesses that can be achieved in one application and the effective drying per mass fraction, since less energy has to be used to evaporate the water. Polyurethane dispersions with high proportions of solids therefore have ecological advantages and are becoming increasingly important. However, these are not easy to manufacture and not possible in every composition.
stabilization
Polyurethanes have a density of around 1.1 g / ml, depending on their composition, and are therefore heavier than water. The tendency to sedimentation and also to coagulation is prevented on the one hand by mutual repulsion of the particles, e.g. B. by ionic groups, and by the viscosity of the liquid medium. Coarse-particle dispersions therefore often contain thickeners and emulsifiers in the aqueous phase, which slow down the settling of the particles accordingly and ensure adequate storage stability. Non-ionic stabilization is achieved by incorporating hydrophilic polyethylene oxide chains into the polymer or as terminal groups; ionic stabilization can be achieved by incorporating anionic groups such as carboxy or sulfonate or cationic and aminic groups. A distinction is therefore made between non-ionic, anionic and cationic polyurethane dispersions.
Cososer
In addition to water, polyurethane dispersions in some cases contain water-dilutable, usually high-boiling organic solvents (cosolvents), frequently N- methylpyrrolidone (NMP), but also glycol ethers in the order of magnitude of 5 to 15 percent by weight. On the one hand, the proportion is often production-related, on the other hand, the cosolvent also enables film formation of hard polyurethanes at room temperature by partially dissolving the surfaces of the dispersion particles after evaporation of the water and subsequent fusion to form a film ( coalescence ). The cosolvent gradually evaporates even without further heat treatment, the film becoming increasingly harder and reaching its final strength. The cosolvents thus contribute to the emission of organic components ( VOC ) and are therefore less desirable. Depending on the content, there are also health and safety-related aspects that make it necessary to take precautionary measures when handling cosolventic polyurethane dispersions. For the co-solvent NMP used, the labeling for preparations was tightened in 2010, so that dispersions containing ≥5% NMP are now classified as reproductive toxic Cat. 1B ( H360D ). This has prompted the manufacturers to largely avoid NMP as a cosolvent or to reduce it to a level of <5% if this is technically possible. As an alternative to NMP, some providers use the less well-known N-ethyl-2-pyrrolidone (NEP). Polyurethane dispersions without cosolvents can, however, achieve coating properties that are comparable to those with cosolvents.
composition
In order that the desired high molar masses can be produced, the polyurethane polymers are preferably built up linearly, ie with as few branches as possible. Otherwise, gel particles would be generated as early as the build-up phase of the polymer , which would hinder the subsequent film formation during use.
The basic building blocks therefore consist of bifunctional subunits and are therefore largely identical to the usual components from which polyurethanes are built, ie isocyanates, polyols and polyamines.
Depending on the isocyanate used, a distinction is therefore made between aliphatic and aromatic polyurethane dispersions. The latter are cheaper, but have the disadvantage of yellowing when exposed to light, with the exception of tetramethylxylylene diisocyanate (TMXDI). The proportion of built-in isocyanate in a polyurethane dispersion is about 8 to 12%.
The polyols make up the largest proportion by mass of the polyurethane and are generally referred to as soft segments . By selecting a polyol with a correspondingly low glass transition temperature , a polyurethane can be produced with corresponding low-temperature flexibility. Here, too, as linearly structured molecules as possible are used, which have two or more terminal hydroxyl groups. Oxidatively hardening , fatty acid-modified polyesters are also of importance . These not only enable good pigment wetting in paint formulations, but also subsequent crosslinking of the dried films to form hard paint layers. The resulting polyurethane dispersions are also called aqueous urethane alkyds due to the origin of the polyesters . Crystallizing polyesters are suitable for thermally activated adhesives. With polyethylene oxide building blocks, a permanent hydrophilicity of the polyurethane can be created, which results in an improved water vapor permeability of the films. Polycarbonate diols are suitable building blocks for extremely impact-resistant behavior, scratch and weather resistance and are therefore important for the finishes of plastic, leather and textile coatings. Ether-stabilized dispersions tend to coagulate at higher temperatures, while ionically hydrophilized dispersions are more stable, but coagulate more easily with the addition of electrolytes . Polyurethane dispersions therefore mostly contain both components for a balanced stability.
Chain extender
The structure of the polymer often takes place in two steps: first, a slightly branched prepolymer is made from the diisocyanates and the polyols. As a result of an excess of diisocyanate, the prepolymers have isocyanates as terminal groups. In the second step, the prepolymers are linked to one another via short-chain diols and / or diamines to form long-chain molecules, often in the presence of water in certain processes during the dispersion. The relationship between generated molar mass, chain extender and reaction with water can be found in the literature.
Functionalizations
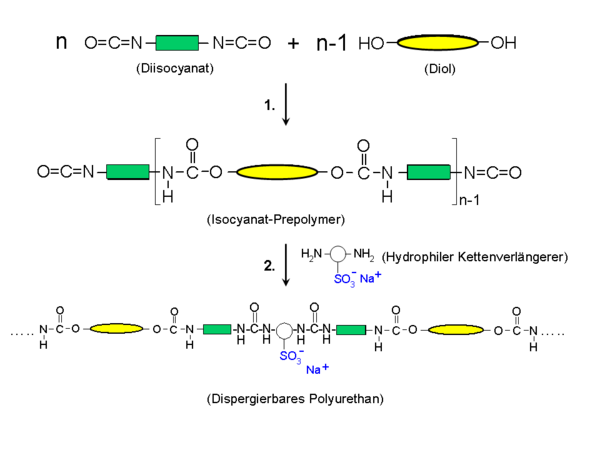
Ionic groups can be built into the polymer via the chain extenders in order to stabilize it as particles dispersed in water. A typical representative is dimethylolpropionic acid (DMPS, English DMPA) for carboxy functionality and diol sulfonates for pendant sulfonic acid groups for anionic polyurethane dispersions. The latter are widespread. The structure of an anionic polyurethane is shown schematically in the diagram below. In the first step, a prepolymer is produced from the aforementioned isocyanates and polyols. In the second step, a hydrophilic group is inserted via the chain extension, using the example of a diamine with a sulfonate group on the side (sodium salt). The polymer obtained is permanently hydrophilic and can be easily dispersed in a suitable process.
To prepare cationic dispersions, quaternary amino functions are incorporated, e.g. B. via methyldiethanolamine . The fine division of a dispersion can primarily be controlled by the number of these hydrophilic groups. There are many, hardly enumerable variations possible to incorporate functional groups. The introduction of terminal, blocked isocyanates is important for heat-activatable crosslinking reactions, but epoxy-modified and silane- modified polyurethanes are also important, as is pendant hydroxyl groups for crosslinking by other reactive agents such as those used in coating formulations.
The modules mentioned above can be combined practically freely. This enables an enormous number of polyurethane dispersions with a wide variety of properties. This also explains the large number of products from different manufacturers and applications. The patent specifications published worldwide are correspondingly extensive. Because of the complexity of the context, patents often contain detailed descriptions of the prior art that are quite useful to the reader. Structure-property relationship and synthesis steps for the production of polyurethanes are also valid for the corresponding dispersions and are comprehensively described in the literature.
Manufacturing
Dispersion in water requires high shear forces in order to obtain finely divided dispersions. One problem is the high viscosities of the isocyanate prepolymer. After the chain extension, the polyurethanes are therefore practically no longer dispersible. Therefore there are two basic possibilities: a) the prepolymer is dispersed directly in water and chain extender under high shear forces and the chain extension takes place in the presence of the aqueous phase, but in the dispersed particles, whereby in addition to the desired reaction also the reaction of water with the isocyanate groups contributes to chain lengthening. To lower the viscosity of the prepolymer, it is heated for dispersion or the co-solvents mentioned are used, which, however, remain in the finished dispersion. There are several variations, such as: B. the melt-dispersing process or the prepolymer mixing process . The latter can also be carried out as a continuous process . b) The complete polyurethane molecule is built up in a water-miscible, low-boiling solvent. The solution is co-dispersed with water and the solvent is finally almost completely distilled off. Acetone has established itself as the solvent of choice, which is why this process is called the acetone process . The advantage of this process lies in the high variability of the starting materials and excellent, finely divided qualities as well as the freedom from organic solvents. The disadvantage is the lower boiler utilization compared to the other processes and the increased expense of acetone recovery. As an alternative to acetone, some manufacturers use 2-butanone (MEK). There is extensive literature on the manufacturing processes and their variants.
Applications
Typical applications are flat applications so that the water and possibly cosolvents can evaporate and leave a polyurethane film. The films can be dried at room temperature, but also at elevated temperatures, if the substrate permits. As soon as the particles touch, when enough water has evaporated, the process is no longer reversible (film formation). High capillary forces arise in the gaps between the particles, so that they lose their phase boundaries, fuse with one another (coalescence) and, after complete drying, form a homogeneous film. The cosolvents used support coalescence and often remain in the film for a longer period of time if it is dried at room temperature. In this case, the cosolvent acts like a plasticizer and the films only reach their final hardness after a while.
The tendency towards foam and skin formation often has a disruptive effect during the transport and filling of the dispersions. A special feature is the targeted generation of foams by mechanical stirring to so-called whipped foams . In areas that are affected by regulations on emissions of organic compounds (VOC), aqueous polyurethane dispersions have largely displaced former solvent-based systems or have already been acquired, e.g. B. industrial and automotive coatings, especially primers, plastic, textile and leather coatings , surface sizing agents for paper, glass fiber sizes, but also coagulation processes for the production of medical gloves and adhesives in the field of shoe, automobile and furniture production. In many cases of applications on textile substrates or adhesives, the polyurethane dispersions are crosslinked shortly before application, such as. B. water-thinnable polyisocyanates added in amounts of 2 to 5% to improve the adhesion to the substrate and the resistances.
Ecological aspects
The stationary, industrial processing of paints and coating materials in Europe has been subject to the VOC directive for the limitation of emissions of volatile organic compounds since 2010, provided that the selected application is described in the directive. In many cases, limit values of 50 mg organic carbon concentration per m³ exhaust air must be observed. It is therefore preferable to use dispersions, and thus also polyurethane dispersions, with very little or no cosolvent content in paints and coatings if no further exhaust air treatment is planned. For technical reasons, it is difficult in individual cases to switch from solvent systems to aqueous formulations, especially when safety aspects have to be observed and there is little experience with new systems.
Polyurethane dispersions in comparison to polyurethane solutions in organic solvents have the disadvantage that about 6 to 7 times more heat of evaporation has to be expended for the water during drying than for conventional solvents. The drying process alone is therefore energetically less favorable for the polyurethane dispersions. However, if one considers the formation of greenhouse gases over the entire process as a carbon footprint , then the polyurethane dispersions turn out to be cheaper than the polyurethane solutions, since the solvents themselves have consumed energy for their production and are either burned directly after drying, e.g. B. in a thermal post-combustion system (TNV) , or get into the atmosphere and are finally oxidized there. Therefore, the carbon in the solvents is ultimately converted to carbon dioxide. For industrial processes, the carbon footprint analysis assumes that solvents in the coating formulations are burned.
literature
- Günter Oertel (Ed.): Polyurethane Handbook. 2nd edition, Hanser Publishers 1993, ISBN 3-446-17198-3 .
Individual evidence
- ↑ T. Michaelis, H. Casselmann, (2009), European Coatings Journal , 2, 38-41.
- ↑ Patent EP1387859 : Polyurethane-polyurea dispersions as a coating agent. Published on July 14, 2010 , inventors: Detlev-Ingo Schütze, Jürgen Urban, Tillmann Hassel, Gerald Kurek, Thorsten Rische.
- ↑ Entry on N-methyl-2-pyrrolidone in the GESTIS substance database of the IFA , accessed on November 10, 2015(JavaScript required) .
- ↑ A. Nasta et al., (2011), European Coatings Journal 07/08, 26-31.
- ↑ Young-Kuk Jhon et al. (2001): Chain extension study of aqueous polyurethane dispersions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 179 (1), 71-78. doi: 10.1016 / S0927-7757 (00) 00714-7
- ↑ Exemplary designs in patent PCT / EP1999 / 00198 .
- ↑ Martin E. Rogers and Timothy E. Long: Synthetic Methods in Step-Growth Polymers , Chapter Polyurethanes and Polyureas, John Wiley & Sons, 2003, ISBN 0-471-38769-X .
- ↑ James W. Rosthauser, Klaus Nachtkamp: Waterborne Polyurethanes . In: Journal of Coated Fabrics . tape 16 , no. 1 , 1986, pp. 39-79 , doi : 10.1177 / 152808378601600103 .
- ^ Ulrich Meier-Westhues, Polyurethane / Lacquer, Adhesives and Sealants , Vincentz Network GmbH & Co. KG Verlag Hannover, 2nd edition (2007), ISBN 3-86630-896-5
- ↑ Dr. Lothar Thiele, Polyurethane Adhesives in Industrial Use - A Location Assessment , Henkel KGaA 2007, ISBN 978-3-923324-19-4 .
- ↑ VOC Solvents Emissions Directive. In: europa.eu. European Commission , April 20, 2016, accessed November 16, 2017 .
- ↑ PFI study on the reduction of VOC in the production of mountain boots ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. (PDF; 1.1 MB).
- ↑ DSM study on the carbon footprints of various paint systems ( Memento of the original from February 22, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 2.1 MB) Retrieved December 8, 2012.