Association (chemistry)
In chemistry, an association is the term used to describe the aggregation of two or more molecules of the same type to form larger groups of molecules, the associations , which were formerly also called supermolecules. The association is brought about by intermolecular forces . Associates can be in the gaseous or liquid aggregate state, but also in solutions.
The association is a special case of aggregation . If the supermolecules consist of different molecules, one speaks of aggregates. The interaction of a molecule with solvent molecules does not fall under the term association, but is called solvation . However, association and solvation can compete with each other.
Hydrogen bonds are often the cause of the formation of associations. Associations of the same number contain stoichiometrically defined association complexes that are in equilibrium with their starting products. In the case of non-equal associations that can only be described statistically, the term “swarm formation” is used.

Examples
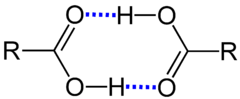
Examples are the agglomeration of several water molecules. Hydrogen fluoride is evenly associated in the gas phase. Carboxylic acids are often in the liquid phase and sometimes also above the boiling point as dimers, for example formic acid and acetic acid . Alcohols, like phenols, form unequal associations.
Another example is ion association , for example when salts are dissolved in aqueous solution.
The hydrophobic association - the agglomeration of organic molecules or parts of molecules in an aqueous environment - is another example. This plays the z. B. plays a fundamental role in biomembrane formation and protein folding . With this type of association, less intermolecular interactions play a role, but an entropy gain is the driving force for the association.
Individual evidence
- ↑ Der Brockhaus, Science and Technology , FA Brockhaus, Mannheim; Spectrum Academic Publishing House, Heidelberg, 2003.
- ↑ Entry on association. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 115.