Siccative
Siccatives (from Latin siccus , 'dry') are substances that are added to oily paints , varnishes , semi-oil and linseed oil varnish in order to accelerate their hardening or setting, usually wrongly referred to as drying .
Chemical reaction
The "drying process" of oil paint is chemically an oxidation , polymerisation and cross-linking with an increase in the molar mass . The "drying" oils first increase in volume, but then shrink again (first wrinkles, then cracks), e.g. B. Linseed oil becomes linoxin . This increases the viscosity of the oil paint. This increase in viscosity is a crosslinking polymerization and not a loss of solvent as is the case with drying . Siccatives act as catalysts in these processes .
Skin formation that is too rapid can be prevented by so-called anti-skinning agents, such as B. butanone oxime can be prevented. Cracks and wrinkles are prevented and the storage stability of the paint is improved.
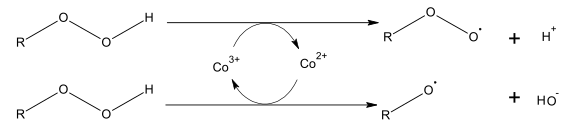
The siccatives used accelerate the breakdown of the peroxides produced during the hardening process. The metal atom is continuously oxidized and reduced, generating a radical in each case. The resulting hydroxide ions and protons combine to form water. The process is illustrated in the following graphic:
Substances used
In the past, harmful materials, such as lead oxide , were used in the production of linseed oil varnish . Today, salts of 2-ethylhexanoic acid , so-called octoates , such as cobalt , manganese and zirconium octoates or the corresponding naphthenates , are mostly used .
Use in oil painting
In oil painting, siccatives are an important component of the paint to accelerate drying. Drying oils are used as the basis of the colorants. The actual oxidation, with and without a siccative, is a continuous process that can take centuries.
By using siccatives, the time it takes for the oil paint to become “nail dry” can be shortened from 5 to 12 days (linseed oil, thin application, depending on the pigments used) to one to two days. With excessive use, premature aging sets in, which is noticeable in strong wrinkling and yellowing , and in the following also in strong cracking. Frequently used siccatives are heavy metal oxides of lead ( Pb ), manganese ( Mn ), cobalt ( Co ), zinc ( Zn ) and the metal salts (= metal soaps ) of mostly unsaturated fatty acids such as oleic acid. Pigments with these heavy metals, such as white lead, have their own siccative effect, which is based on saponification reactions with an increase in viscosity.
In practice, mixtures of different metal soaps are often used. Solutions of siccatives in oil - sometimes called siccative extracts - often turn cloudy after standing for a while. They are then allowed to settle in open vessels for clarification.
“A red lead siccative is obtained if you boil linseed oil varnish with red lead and umber while stirring continuously until a pulp-like mass is formed, and this is then diluted with turpentine oil . The clear varnish is poured off from the sediment after a few days.
For zinc white paints, boil linseed oil with 5% brown stone powder , which is sewn into a sack of canvas that is attached to the kettle so that it does not touch the floor. Boil twice for 10 to 12 hours and then dilute with turpentine oil. The dark brown liquid obtained gives large quantities of oil and varnish the ability to dry quickly.
Most often one uses boric acid manganese oxide , which is rubbed in with a little linseed oil and boiled once with about 300 to 400 parts of linseed oil. Zinc white , mixed with 5% boric acid manganese oxide, is sold as Siccatif zumatique and makes linseed oil varnish paints dry faster if you add 2.5% of the same to them. Solutions of shellac in ammonia or in borax solution are also used as a siccative. The use of siccatives is particularly advisable for earth colors , ultramarine and zinc white , superfluous when painting with lead white , red lead , chrome yellow , as these colorants already have a drying effect. "
Health notice
The heavy metal ions contained in most siccatives (cations of lead, manganese, cobalt, zinc etc.) are toxicologically not harmless.
Individual evidence
- ↑ a b Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1287.
- ↑ Entry on skin contraceptives. In: Römpp Online . Georg Thieme Verlag, accessed on April 6, 2016.
- ↑ Bernd Strehmel; Peter Mischke; Michael Groteklaes; Thomas Brock: Textbook of paint technology . 4th revised edition. Vincentz Network, [p. l.], ISBN 3-86630-815-9 , pp. 203 .
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 5: Pl-S. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1987, ISBN 3-440-04515-3 , pp. 3772-3773.