Manganese (II) oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
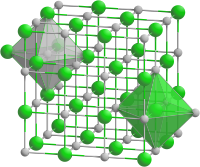
|
||||||||||||||||
| __ Mn 2+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Manganese (II) oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | MnO | |||||||||||||||
| Brief description |
green powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 70.94 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.45 g cm −3 |
|||||||||||||||
| Melting point |
1650 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
0.5 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Manganese (II) oxide is one of the oxides of manganese with the formula MnO.
Occurrence
Manganese oxide occurs naturally in the mineral manganosite .
Extraction and presentation
Manganese (II) oxide is by reduction of manganese (IV) oxide -containing ores with natural gas or coal at temperatures between 400 and 1000 ° C. Manganese (II) oxide formed must be cooled under protective gas in order to avoid reoxidation to tetravalent manganese.
Manganese (II) oxide can also be obtained by heating manganese (II) carbonate MnCO 3 in a vacuum:
properties
Manganese (II) oxide crystallizes in the cubic sodium chloride structure . MnO is an antiferromagnetic insulator at low temperatures , the magnetic structure of which was one of the first compounds to be elucidated. For this purpose, neutron diffraction experiments were carried out as early as 1951 , which showed that the magnetic moments of Mn 2+ each align against those of all of its neighbors. That is the meaning of antiferromagnetism: the magnetic moments are aligned with each other and thus cancel each other out, the material initially appears non-magnetic. However, this alignment also means that all magnetic moments are aligned parallel to one another along the spatial diagonal . At very low temperatures, this leads to a slight rhombohedral distortion precisely along this direction, which is denoted by [111] . Above the order temperature of 110 K, known as the Néel temperature , manganese (II) oxide becomes paramagnetic .
use
Manganese (II) oxide is used as a component of casting powder in the metalworking industry, as a fertilizer additive or feed additive, and as a green color pigment in fabric printing .
safety instructions
Powdered, freshly produced manganese (II) oxide can self-ignite in air.
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 1344-43-0 in the GESTIS substance database of the IFA , accessed on May 9, 2017(JavaScript required) .
- ^ National Pollutant Inventory: Manganese & compounds. accessed on February 17, 2015.
- ↑ Manganosit (Mineral Atlas)
- ↑ The discovery of manganosite in the Harz Mountains (Stollen Troll)
- ↑ WH McCarroll: Oxides- solid state chemistry. In: R. Bruce King (Ed.): Encyclopedia of Inorganic chemistry. John Wiley & Sons, 1994, ISBN 0-471-93620-0 .
- ↑ JE Greedon: Magnetic oxides. In: R. Bruce King (Ed.): Encyclopedia of Inorganic chemistry. John Wiley & Sons, 1994, ISBN 0-471-93620-0 .
- ↑ CG Shull, WA Strauser, EO Wollan: Neutron Diffraction by Paramagnetic and Anti Ferromagnetic Substances. In: Phys. Rev. Volume 83, 1951, pp. 333-345. doi: 10.1103 / PhysRev.83.333
- ↑ G. Gigacher, Ch. Bernhard, W. Kriegner: Properties of high-manganese steels under conditions similar to continuous casting. ( Memento from September 29, 2007 in the Internet Archive )
- ↑ Manganese and its compounds (University of Regensburg)


