Dicobalt octacarbonyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
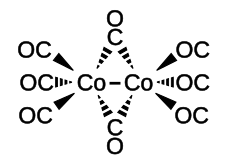
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Dicobalt octacarbonyl | |||||||||||||||
| Molecular formula | [Co 2 (CO) 8 ] | |||||||||||||||
| Brief description |
orange crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 341.95 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.78 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
from 52 ° C decomposition |
|||||||||||||||
| Vapor pressure |
0.7 mmHg (25 ° C) |
|||||||||||||||
| solubility |
very bad in water (<0.1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Dicobalt octacarbonyl is a binuclear complex in which two cobalt nuclei are surrounded by eight carbonyl ligands.
presentation
The complex can be produced by the reaction of cobalt (II) salts (e.g. cobalt (II) acetate or cobalt (II) carbonate ) under high carbon monoxide pressure. Often this reaction is carried out in the presence of cyanide salts .
properties
Dicobalt octacarbonyl is an orange solid (larger crystals are red) which decomposes at 52 ° C to tetracobalt dodecacarbonyl [Co 4 (CO) 12 ]. Even at room temperature, the complex slowly decomposes in air, releasing carbon monoxide.
The complex is in equilibrium between two structures, one of which consists of two trigonal-bipyramidal -coordinated cobalt nuclei with a bond between the cobalt atoms, the other of which consists of a complex bridged by two carbonyl ligands. The equilibrium is on the side of the bridged complex species. The bond length between the cobalt nuclei in the bridged complex is 252 pm , the Co – C bond to the terminal carbonyls is 180 pm, to the bridging carbonyls 190 pm.
use
Dicobalt octacarbonyl is required in the Pauson-Khand reaction for the synthesis of cyclopentenones. By splitting off two carbonyl ligands, it provides the cobalt species that is used for addition to the alkyne used. The same applies to the Nicholas reaction , for which the same cobalt species is required.
The mononuclear complex [CoH (CO) 4 ] is formed by hydrogenation of the complex .
This complex is used as a catalyst for hydroformylation . Here, dicobalt octacarbonyl is reacted with hydrogen , so that cobalt carbonyl hydride is formed as a catalytically active species.
The technical synthesis of phenylpyruvic acid uses the compound as a catalyst in the double carbonylation of benzyl chloride using carbon monoxide and water .
[Co (CO) 4 ] - ions can be formed by reduction with elemental alkali metals .
literature
- Christoph Elschenbroich : Organometallic chemistry . (= Teubner study books chemistry ). 5th revised edition. Teubner, Wiesbaden 2005, ISBN 3-519-53501-7 .
- Lutz H. Gade: coordination chemistry . Wiley-VCH, Weinheim et al. 1998, ISBN 3-527-29503-8 .
Individual evidence
- ↑ Entry on cobalt carbonyls. In: Römpp Online . Georg Thieme Verlag, accessed on December 13, 2015.
- ↑ a b c d e f g Entry on dicobalt octacarbonyl in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens, 5th Edition . William Andrew, 2008, ISBN 978-0-8155-1904-1 , pp. 697 ( limited preview in Google Book search).
- ↑ Data sheet di-Cobaltoctacarbonyl (PDF) from Merck , accessed on March 24, 2011.
- ↑ Georg Brauer (ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1833.
- ^ GG Sumner, HP Klug, LE Alexander: The crystal structure of dicobalt octacarbonyl. In: Acta Cryst. 1964, 17, pp. 732-742, doi : 10.1107 / S0365110X64001803 .
- ↑ Christoph Elschenbroich: Organometallchemie. 6th edition. Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 633-637.
- ↑ W. Bertleff, M. Roeper, X. Sava: Carbonylation. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2007. doi : 10.1002 / 14356007.a05_217.pub2







![{\ displaystyle \ mathrm {[Co_ {2} (CO) _ {8}] \ + \ H_ {2} \ longrightarrow \ 2 \ [CoH (CO) _ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a4f80426e35289df4aa70d29aa181a9541c9ca69)
![{\ displaystyle \ mathrm {[Co_ {2} (CO) _ {8}] \ + \ 2 \ Na \ longrightarrow \ 2 \ Na [Co (CO) _ {4}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b394ef88a0038694e0d2d227b0087bef2fc68f96)