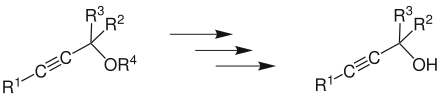
Nicholas reaction
The Nicholas reaction is a chemical reaction that converts stabilized propargylic cations with nucleophiles . The triple bond of the propargyl alcohol or ether used is protected from the formation of the cation by dicobalt hexacarbonyl . Alkylated alkynes can be obtained by the Nicholas reaction .
mechanism
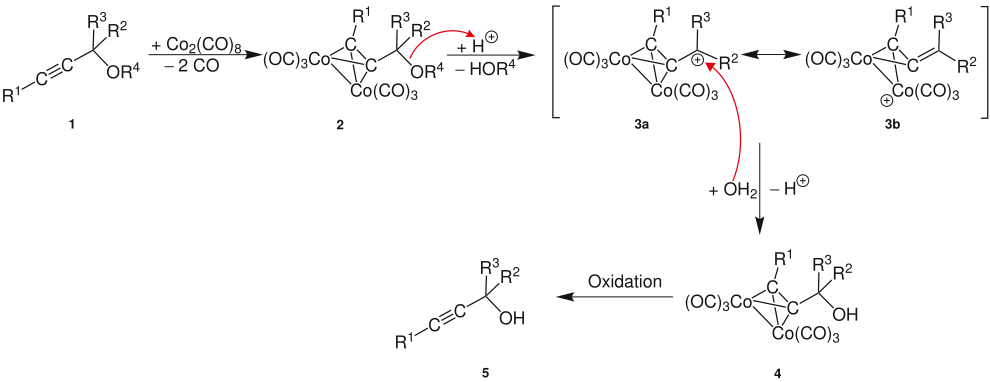
Propargyl cations form two mesomeric border structures, so that a nucleophile can attack at two different positions and no uniform product is formed.
A propargyl ether or a propargyl alcohol (R 4 = H) forms a propargyl cation under acid catalysis. This is mesomeric stabilized (boundary structures a and b ) and allows nucleophilic attack at two different positions (R 1 to R 3 alkyl or aryl radicals).
In order to increase the selectivity for the desired alkylated alkyne, the triple bond in the Nicholas reaction is first protected by reaction with dicobalt hexacarbonyl ( 2 ). Dicobalt hexacarbonyl can be obtained from dicobalt octacarbonyl . The cation is now formed from the alcohol or the ether by reaction with a Lewis acid ( 3a and 3b ). Since the triple bond is protected, the mesomeric boundary structures shown in the above scheme can no longer form. The nucleophile only has one position left to attack ( 3a ). Thus, only the desired alkylated alkyne 5 is obtained, which is released by subsequent oxidative cleavage of the cobalt complex .
See also
swell
- KM Nicholas, R. Pettit: An alkyne protecting group , in: Tetrahedron Letters , 1971 , 37 , pp. 3475-3478; doi : 10.1016 / S0040-4039 (01) 97209-0 .
- KM Nicholas, R. Pettit: On the stability of α- (alkynyl) dicobalt hexacarbonyl carbonium ions , in: J. Organomet. Chem. , 1972 , 44 , C21-C24; doi : 10.1016 / 0022-328X (72) 80037-8 .
- RE Connor, KM Nicholas: Isolation, characterization, and stability of α - [(ethynyl) dicobalt hexacarbonyl] carbonium ions , in: J. Organomet. Chem. , 1977 , 125 , C45-C48, doi : 10.1016 / S0022-328X (00) 89454-1 .
- RF Lockwood, KM Nicholas: Transition metal-stabilized carbenium ions as synthetic intermediates. I. α - [(alkynyl) dicobalt hexacarbonyl] carbenium ions as propargylating agents , in: Tetrahedron Letters , 1977 , 18 , pp. 4163-4166; doi : 10.1016 / S0040-4039 (01) 83455-9 .
- BJ Teobald: The Nicholas reaction: the use of dicobalt hexacarbonyl-stabilized propargylic cations in synthesis , in: Tetrahedron , 2002 , 58 , pp. 4133-4170. doi : 10.1016 / S0040-4020 (02) 00315-0 .