Shell Higher Olefin Process
The Shell Higher Olefin Process (SHOP, often incorrectly also SHOP process) is a chemical process for the production of linear α-olefins by ethene oligomerization, isomerization and ethenolysis , which was invented and introduced by the Royal Dutch Shell . It is the first example of an industrially used heterogenization of a homogeneous catalyst .
history
The process was introduced in 1977 and today has a capacity of around 1 million tons per year. The process goes back to the work of Karl Ziegler and Günther Wilke at the Max Planck Institute for Coal Research in Mülheim an der Ruhr . Wilhelm Keim modified the nickel catalyst by introducing a phosphorus-oxygen ligand. Further research at the Shell research laboratories in Emeryville and Amsterdam led to the development of the process. The first commercial plant went into operation in 1977 in Geismar .
Technical realization
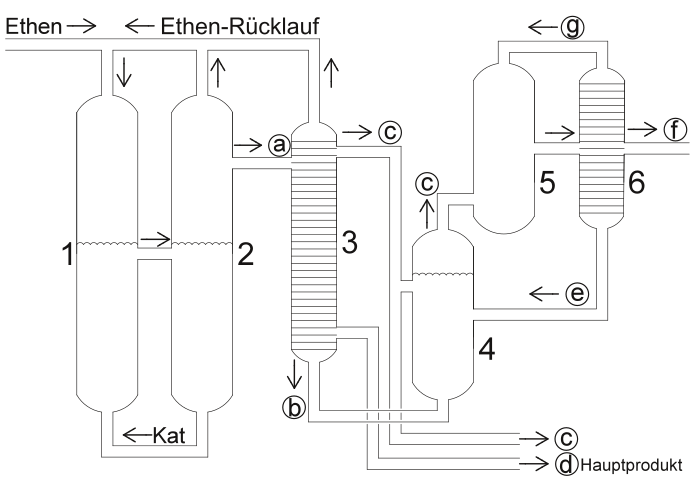
In the course of the shop process, six different fractions with different compositions arise. These fractions (a – f) are marked accordingly in the system drawing. Fractions c, d and f are taken from the process and used industrially. Fractions a, b and e are not industrially relevant, but can be returned to the process and converted to the other fractions. The respective chain lengths can be assigned using the following table:
| fraction | shortest chain length |
longest chain length |
comment |
|---|---|---|---|
| a | 4th | ∞ | only α – olefins with an even number of carbon atoms |
| b | 20th | ∞ | only α – olefins with an even number of carbon atoms |
| c | 4th | 10 | only α – olefins with an even number of carbon atoms |
| d | 12 | 18th | only α – olefins with an even number of carbon atoms |
| e | 14th | ∞ | Olefins with intermediate double bonds; an odd number of carbon atoms is also possible |
| f | 11 | 14th | Olefins with intermediate double bonds; an odd number of carbon atoms is also possible |
| G | 4th | 10 | Olefins with intermediate double bonds; an odd number of carbon atoms is also possible |
The system for the SHOP process is divided into six different components. In addition to two distillation chambers (3) and (6) for the separation into different fractions, the system consists of the actual oligomerization reactor (1), the separator (2), the isomerization reactor (4) and the reactor for metathesis (5).
Oligomerization and separation
In the oligomerization reactor (1), ethene is converted into longer-chain α-olefins in the presence of a liquid catalyst phase of a nickel-phosphine complex. Here, a particularly pure form of alkenes is formed, each of which has only one double bond. The proportion of α-olefins is over 95 percent. The product mixture is separated from the catalyst phase in the separator (2), washed with fresh solvent and then passed into the distillation column (3). Excess ethene is fed back into the reactor (1).
At the end of the first distillation, three fractions leave the chamber. The fraction of products with chain lengths between twelve and 18 carbon atoms are discharged from the process and processed further in the industry. They are the main products of the SHOP process. Some of the chain lengths between four and ten carbon atoms are removed and some are fed into the isomerization reactor (4). The fraction of the α-olefins which have more than 20 carbon atoms are completely fed into the isomerization reactor (4).
Pressure and temperature
In the first step, the ethene oligomerization takes place at temperatures of 80 to 120 ° C. and a pressure of 70 to 140 bar (7 to 14 MPa).
catalyst
The reaction is carried a nickel - phosphine - complex of the type (C 6 H 5 ) 2 P (CH 2 ) 2 COONi with Diphenylphosphinoessigsäure or 2-diphenylphosphinobenzoic acid catalyzed as a ligand. This step is carried out in a polar solvent such as 1,4-butanediol . Since the α-olefins formed are not soluble in them, they can easily be separated off. This creates a mixture of even-numbered α-olefins with a Schulz-Flory distribution .
Isomerization
During isomerization, the α – olefins are converted into compounds that have double bonds in the middle. In this process step, the chain length of the molecules remains constant, only the position of the double bond is changed. The reaction takes place in dissolved magnesium oxide catalyst under mild reaction conditions. The temperature is between 80 ° C and 140 ° C and the pressure between three bar and 20 bar.
metathesis
During the subsequent metathesis, low and high molecular weight olefins become a mixture of alkenes with a new distribution of chain lengths. Only in this process can chain lengths with an uneven number of carbon atoms arise. Only chain lengths of eleven to 14 carbon atoms are relevant for industry. The practical thing about the SHOP process is that the products of other chain lengths do not arise as waste products, but can be fed back into the process. In this way, shorter molecules can be fed back into the metathesis reactor (5) and longer-chain molecules are fed into the isomerization reactor (4).
Products
By combining oligomerization, isomerization and metathesis, it is possible to convert almost all of the ethene used into the desired product fractions. The α-olefins are converted into fatty alcohols by hydroformylation . These can be converted into fatty alcohol sulfates by direct sulfation . The reaction with ethylene oxide produces fatty alcohol ethoxylates , which are converted either as nonionic surfactants or, after sulfation, into anionic surfactants.
The α-olefins are used as co- monomers in the production of linear low density polyethylene (LLD-PE).
mechanism
The process and its chemistry were studied intensively in the working group of Professor Wilhelm Keim at RWTH Aachen University , who is considered to be one of the key figures in the development of the process.
The active catalyst is nickel hydride, which is formed by splitting off the cyclooctadiene ligand from the starter complex. By inserting ethene into the nickel-hydrogen bond, oligomers are formed which, through β-elimination, are α-olefins of various chain lengths, but always with an even number of carbon atoms. It is assumed that the diphenylphosphinoacetic acid ligand influences the chain length distribution of the resulting olefins.
literature
- Wilhelm Keim: Oligomerization of ethene to α-olefins: Invention and development of the Shell higher olefin process (SHOP). In: Angewandte Chemie. 125, 2013, pp. 12722–12726, doi : 10.1002 / anie.201305308 .
- Ulfert Onken, Arno Behr: Chemical process science - textbook of technical chemistry . Volume 3, 1st edition, Georg Thieme Verlag, Stuttgart 1996.
Individual evidence
- ↑ a b Dieter Vogt : SHOP Process . In: Boy Cornils , Wolfgang A. Herrmann : Aqueous-Phase Organometallic Catalysis , John Wiley & Sons, 1998, ISBN 3-527-29478-3 , pp. 541-547.
- ^ Paul CJ Kamer, Piet WNM van Leeuwen: Phosphorus (III) Ligands in Homogeneous Catalysis: Design and Synthesis , 566 pages, John Wiley & Sons, 2012, ISBN 0-470-66627-7 .
- ↑ Catalysis: Concepts and Green Applications, by Gadi Rothenberg . books.google.de. Retrieved December 11, 2009.