Markovnikov's rule
The Markovnikov rule (after Vladimir Markovnikov ) describes in organic chemistry , the products of electrophilic addition of hydrogen halides to carbon -Kohlenstoff- double bonds in unsymmetrical alkenes .
The rule states that when hydrogen halides are added to asymmetric alkenes, the hydrogen atom is always bound to the carbon atom that is already richer in hydrogen. The halogen atom is accordingly bonded to the lower hydrogen, i.e. more highly substituted, carbon atom. The same effect can be observed with the addition of water: The H atom is bonded to the lower substituted carbon atom, the OH group to the higher substituted one. In general, one can say that + δ electrophile - nucleophile δ− follow this rule (exceptions: see below). The motto is: "He who has will be given."
Explanation
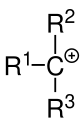
The reason for this process is that in an electrophilic addition, a positively charged and as well stabilized as possible carbenium ion is formed as an intermediate stage . If the positive charge is on a tertiary carbon atom, it is stabilized by three + I effects of the adjacent alkyl radicals. A secondary carbon atom is only stabilized by two + I effects and a primary by one. In addition to + I effects, hyperconjugation also stabilizes the carbenium ion. Therefore, tertiary carbenium ions are lower in energy and therefore more stable than secondary ones, and these in turn are more stable than primary carbenium ions. Almost exclusively tertiary carbenium ions are formed.
example
In the following reaction, 2-methylprop-1-ene reacts with hydrogen chloride:
According to route a), the hydrogen atom is added to the carbon atom, which is less substituted, so that a more stable tertiary carbenium ion is formed. This creates the so-called Markovnikov product after the addition of the chloride ion, as this was created according to Markovnikov's rule. In path b) the hydrogen atom is bound to the more highly substituted carbon atom, so that a labile primary carbenium ion would be formed and then the anti-Markovnikov product.
Since the intermediate product in route a) is more stable, this is preferentially formed.
Exceptions
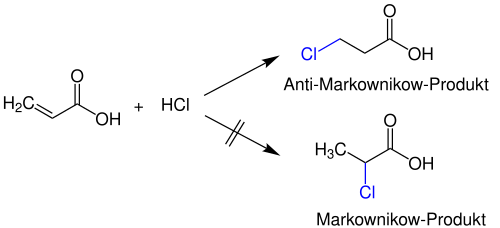
However, if the stability of the two possible carbenium ions in the transition state is not determined exclusively by the + I effects of alkyl substituents , anti-Markovnikov products can also occur. For example, when adding HCl to acrylic acid (CH 2 = CH – COOH), only 3-chloropropionic acid is obtained , because the more stable carbenium ion is not formed in the immediate vicinity of the electron-withdrawing carboxy group ( −I effect ) during the reaction .
In the radical addition , the hydroboration and hydrosilylation the Markownikowregel also does not apply (as first Morris S. Kharasch showed). Again, anti-Markovnikov products are obtained in which the heteroatom is bonded to the lower substituted carbon atom.
If alkenes are formed from the Markovnikov product of the primary addition, the direction ( regioselectivity ) of the elimination can be described in more detail using the terms Saytzeff rule and Hofmann product .
criticism
The application of the historically important Markovnikov rule leads to incorrect predictions about the expected addition products to unsymmetrical alkenes if mesomeric-stabilized carbenium ions can form as intermediates . The mesomeric effects override (overcompensate) the inductive effects. The carbenium ion that is more stable is always formed as an intermediate product. This then automatically results in the direction of addition.
Individual evidence
- ^ A b c Milton Orchin, Roger S. Macomber, Allan R. Pinhas, R. Marshall Wilson: The Vocabulary and Concepts of Organic Chemistry. 2nd edition, John Wiley & Sons, Hoboken 2005, ISBN 0-471-68028-1 , pp. 558-559.
- ↑ a b Paula Yurkanis Bruice: Organic Chemistry. 4th edition, Prentice-Hall, 2003, ISBN 0-131-41010-5 , pp. 148-149.
- ↑ Matthias Beller, Jayasree Seayad, Annegret Tillack, Haijun Jiao: Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes . In: Angewandte Chemie . tape 116 , no. 26 , June 28, 2004, ISSN 0044-8249 , p. 3448–3479 , doi : 10.1002 / anie.200300616 ( wiley.com [accessed June 4, 2019]).