Hydroboration
The hydroboration is a reaction of organic chemistry and was of Herbert C. Brown discovered. It is used to functionalize alkenes . The hydroboration of alkenes with borane yields an alkyl borane . Alkylboranes are important intermediates because they can be easily oxidized or halogenated.
Overview
The reaction of a terminal alkene with borane results in the formation of the alkyl borane 1 through addition . The dialkylborane 2 is formed by adding another molecule of the alkene :
The trialkylborane 3 is formed by adding a third molecule of the alkene :
The alkylboranes 1 , 2 , 3 can then be converted to primary alcohols with hydrogen peroxide and sodium hydroxide solution :
How many molecules of the primary alcohol can be synthesized from one molecule of borane depends on the number of alkyl radicals in the borane.
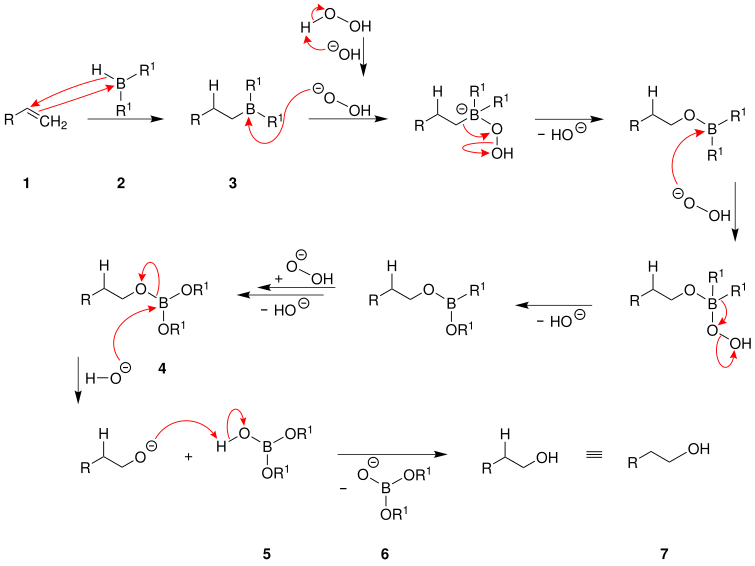
Reaction mechanism
A suggestion for the reaction mechanism:
The alkene 1 is converted to the more highly substituted borane 3 by addition of the borane 2 . The alkyl groups of borane 3 are converted into alcoholate groups by triple reaction with hydroperoxide anions and subsequent elimination of one hydroxide ion in each case . If R 1 stands for a hydrogen atom , the hydride groups are converted into hydroxyl groups instead . This creates the boric acid ester 4 . The resulting boric acid ester 4 is then hydrolyzed under alkaline conditions to give boric acid dialkyl ester 5 by adding hydroxide ions . The resulting alcoholate anion is protonated by the boric acid dialkyl ester 5 and this is how the primary alcohol 7 is formed . In addition, the ion 6 is formed . If the OR 1 groups of ion 6 are alcoholate groups, these can also be split off by adding hydroxide ions and converted into alcohols by protonation .
literature
- Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 .
- FA Carey, RJ Sundberg: Organic Chemistry , VCH, Weinheim 1995, ISBN 3-527-29217-9 .
- HC Brown, Tetrahedron 1961 , 12 , 117
- G. Zweifel and HC Brown: Hydration of Olefins, Dienes, and Acetylenes via Hydroboration in Organic Reactions Vol. 13 , Wiley 1963, doi : 10.1002 / 0471264180.or013.01