Hydrosilylation
The hydrosilylation refers to the syn -selective anti-Markovnikov addition of a silane to a double bond. The reaction was discovered by Speier in the 1950s. In contrast to, for example, the hydroboration , the reaction does not take place without a catalyst.
Catalysts
Platinum catalysts are often used in which the active species is always present as platinum (0). Hexachloridoplatinic acid in isopropanol can serve as a precatalyst (Speier catalyst). If this catalyst system is reacted with additives such as 1,1,3,3-tetramethyl-1,3-divinyldisiloxane, it is called the Karstedt catalyst. Both dinuclear platinum (0) alkene complexes and colloidal platinum have been detected in mixtures of this type.
mechanism
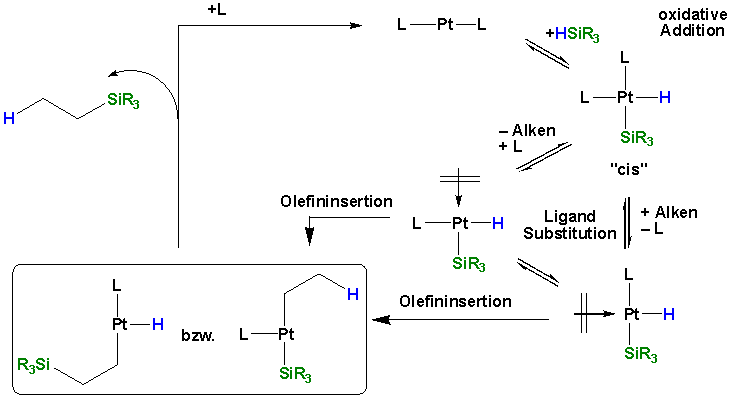
The mechanism of hydrosilylation consists of oxidative addition of the silane to the Pt (0) species, olefin coordination with substitution of a ligand, olefin insertion and reductive elimination.
Asymmetric hydrosilylation
The hydrosilylation can also be carried out asymmetrically using palladium catalysts. For example, the chiral ligand 2-diphenylphosphino-2'-methoxy-1,1'-binaphthyl ("MeO-MOP"), which can be synthesized from BINOL, can be used for this purpose.
Hydrosilylation on alkynes
In the hydrosilylation of alkynes i. A. does not produce high regio- and stereoselectivities, but the vinylsilanes accessible in this way can be used in subsequent reactions, such as, for example, the Hiyama coupling .
Individual evidence
- ↑ a b c Dirk Steinborn "Fundamentals of organometallic complex catalysis", p. 245 ff, 1st edition, ISBN 978-3-8351-0088-6 .
- ↑ Qingle Zenga, Hui Zenga & Zhiren Yanga, "New Route for Synthesis of MeO-MOP", Synthetic Communications 2011 , 41, 23, 3556-3560, doi : 10.1080 / 00397911.2010.519095 .