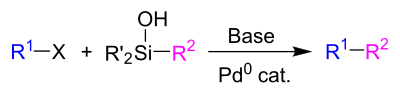Hiyama clutch
The Hiyama coupling is a name reaction in organic chemistry that was discovered in 1988 by the Japanese chemists Tamejiro Hiyama (* 1946) and Yasuo Hatanaka. It is a palladium- catalyzed cross-coupling reaction in which an aryl, alkyl or vinyl halide is reacted with an organosilane to form a CC bond.
The radical X is usually the halides chloride (Cl), bromide (Br) or iodide (I), but other leaving groups such as triflate (OTf) are also possible. The radicals R 'on the silicon atom can be fluoride or chloride, but also other alkyl radicals or alkoxy groups . As a fluoride source, for example, tetrabutylammonium fluoride (TBAF) in stoichiometric amounts and as a palladium source, for example, palladium (II) acetate (Pd (OAc) 2 ) or tris (dibenzylidene acetone) dipalladium (0) (Pd 2 (dba) 3 ) in catalytic amounts .
Reaction mechanism
The reaction mechanism of the Hiyama coupling is similar to that of other cross-coupling reactions, such as the Suzuki coupling or the Negishi coupling . The essential point of the mechanism is a catalytic cycle, which is exemplified below with a trifluorosilyl- substituted compound (R 2 -SiF 3 ).
The catalytic cycle begins with the catalytically active, two-coordinate Pd 0 complex 2 . Since this cannot be used directly, it must first be prepared in situ from a precatalyst 1 . This takes place via the equilibrium reaction A , which occasionally, depending on the compound used, only takes place after the addition of a further ligand (for example triphenylphosphine (PPh 3 )). The next step is an oxidative addition ( B ) of the halide 3 used as starting material . This results in a four-coordinate Pd II complex, which is initially cis -substituted, but isomerizes quickly to trans -complex 4 as a rule . Before the next step of the cycle takes place, the reaction partner of this step must be generated. This is done by adding a fluoride to the trifluorosilyl compound 5 used here as starting material, a negatively charged, pentavalent silicon intermediate 6 being formed. Due to the chemical equilibrium D, there is always a small amount of the R 2 anion 7 which can enter the catalytic cycle. A ligand substitution occurs ( C ), in which the halide ligand is exchanged for the radical R 2 ( 8 ). The original C-Si bond (R 2 -Si) is now a C-Pd bond (R 2 -Pd), so a transmetalation has taken place. After a cis - trans isomerization ( E ), a reductive elimination ( F ) can take place on complex 9 , in which the coupling product 10 and again the active catalyst 2 are formed.
Hiyama Denmark coupling
A variant of the reaction that takes place under fluoride-free conditions is the Hiyama-Denmark coupling named after Hiyama and Scott Denmark . An organosilanol is used as the coupling partner . Instead of fluoride, a Brønstedt base can be used to activate this compound.
Mechanistically, this reaction proceeds in a similar way to the fluoride-activated standard variant of the coupling. Activation takes place here, however, by deprotonation of the silanol with formation of the silanolate, which instead of the R 2 anion coordinates to the Pd center with the formation of a Pd-O bond. The radical R 2 to be coupled then coordinates to the palladium center, the silanone R ' 2 Si = O, being eliminated, which reacts immediately with another equivalent to form a siloxane .
See also
literature
- Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr., Name Reactions and Reagents in Organic Synthesis , John Wiley, Hoboken (NJ) 2005, ISBN 0-471-22854-0 , pp. 320-321.
- Cristina Mateo, Carolina Fernández-Rivas, Antonio M. Echavarren, Diego J. Cárdenas, Isolation of the Transmetalation Step in the Hiyama Cross-Coupling Reaction of Organosilanes . In: Organometallics 16, 1997, 1997-1999.
Individual evidence
- ↑ Yasuo Hatanaka, Tamejiro Hiyama: Cross-coupling of organosilanes with organic halides mediated by a palladium catalyst and tris (diethylamino) sulfonium difluorotrimethylsilicate . In: Journal of Organic Chemistry 53, 1988, 918-920, doi: 10.1021 / jo00239a056 .
- ↑ a b Shaundra Riggleman, Philip DeShong: Application of Silicon-Based Cross-Coupling Technology to triflate . In: Journal of Organic Chemistry 68, 2003, 8106-8109, doi: 10.1021 / jo034809g .
- ↑ Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr .: Name Reactions and Reagents in Organic Synthesis . John Wiley, Hoboken (NJ) 2005, ISBN 0-471-22854-0 , pp. 320-321 .
- ↑ Arturo L. Casado, Pablo Espinet: On the Configuration Resulting from Oxidative Addition of RX to Pd (PPh 3 ) 4 and the Mechanism of the cis-to-trans Isomerization of [PdRX (PPh 3 ) 2 ] Complexes (R = Aryl , X = halide) . In: Organometallics 17, 1998, 954-959, doi: 10.1021 / om9709502 .
- ↑ a b Scott E. Denmark, Christopher S. rain: Palladium-Catalyzed Cross-Coupling Reactions of organosilanol and Their Salts . In: Accounts of Chemical Research 41, 2008, 1486-1499, doi: 10.1021 / ar800037p .