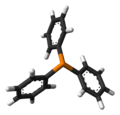
Triphenylphosphine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
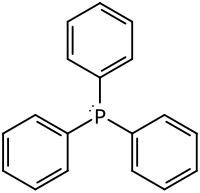
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenylphosphine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 15 P | |||||||||||||||
| Brief description |
colorless, monoclinic, triboluminescent prisms |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 262.28 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.19 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
80 ° C |
|||||||||||||||
| boiling point |
360 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.6358 (80 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 5 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Triphenylphosphine (also: triphenylphosphine, triphenylphosphorus, phosphorus triphenyl) is a ligand (complexing agent) for the production of metal complexes that are required in chemical synthesis .
properties
Triphenylphosphine forms colorless, characteristically smelling crystals with a melting point of 80 ° C and boiling point of 360 ° C, which are not soluble in water, but dissolve quite well in organic solvents . Triphenylphosphine easily forms transition metal complexes that dissolve in organic solvents and are often valuable for synthesis. Triphenylphosphine oxide is formed over time in air .
Manufacturing
On a laboratory scale, triphenylphosphine can be represented by the action of phenylmagnesium bromide or phenyllithium on phosphorus trichloride . Industrial production takes place from phosphorus trichloride, chlorobenzene and sodium .
use
Triphenylphosphine is used to produce the Wittig reagent , which is required on an industrial scale for the synthesis of vitamin A (retinol) and olefins . Another important industrial build-up reaction is the modern variant of hydroformylation (also: oxo synthesis ), in which a rhodium complex of triphenylphosphine is used; alkenes react with carbon monoxide and hydrogen to form valuable aldehydes . These are mostly processed into important alcohols . On a laboratory scale, triphenylphosphine is used to prepare esters , ethers , amides and thioethers in the Mitsunobu reaction . In addition, triphenylphosphine is used in the laboratory as part of the Staudinger reaction , in which primary amines are synthesized from azides . Triphenylphosphine also plays a role as a ligand of the palladium catalyst, for example in the Heck reaction or the Sonogashira coupling (such as (PPh 3 ) 2 PdCl 2 or (PPh 3 ) Pd). Sometimes the arsenic analog triphenylarsine is also used there.
Triphenylphosphine can be used as an adjuvant in tannery processes to remove horny substances from animal hides.
structure
Rod model of triphenylphosphine.
Space-filling model of triphenylphosphine.
Individual evidence
- ↑ Entry on TRIPHENYLPHOSPHINE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c Entry on triphenylphosphine. In: Römpp Online . Georg Thieme Verlag, accessed on May 3, 2014.
- ↑ a b c d e f Entry on triphenylphosphine in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-512.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 603-35-0 or triphenylphosphine ), accessed on November 2, 2015.
- ↑ Entry on triphenylphosphine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ J. Dodonow, H. Medox: For the knowledge of the Grignard reaction: About the representation of tetraphenyl-phosphonium salts . In: Reports of the German Chemical Society . tape 61 , no. 5 , 1928, pp. 907 - 911 , doi : 10.1002 / cber.19280610505 .
- ^ DEC Corbridge: Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology . 5th edition. Elsevier, Amsterdam 1995, ISBN 0-444-89307-5 .
- ↑ Procedure for removing horny substances from animal hides