Phosphine ligands

Phosphine ligands are phosphines , ie compounds of formula PRR'R "(R, R ', R" = H, alkyl, aryl, etc.) used as ligands in metal complexes , and often associated with organometallic chemistry or homogeneous catalysis are used However, finding they are also used in other areas of chemistry.
Ordinary phosphines
The most common phosphine ligands are of type PR 3 . They are three times substituted with the same radicals. Some of the most common of them are triphenylphosphine and trimethylphosphine . The triarylphosphines are usually stable solids, whereas the trialkylphosphines are liquids that react in air to form their corresponding phosphine oxides (R 3 PO). Such ligands are classified based on their donor strength and steric demand. A quantification is carried out using the Tolman Electronic parameter and the Tolman cone angle . In general, alkylphosphines are stronger bases and σ donors.
Multidentate phosphines
Bidentate phosphines
Common bidentate, chelating phosphine ligands are, for example, dppe and dmpe with the structure R 2 PCH 2 CH 2 PR 2 (R = Ph, Me).
Tridentate phosphines
There are two types of tridentate triphosphines, linear and tripodal. Both are called (confusingly) triphos . The phenyl-substituted variants have the formulas PhP (CH 2 CH 2 PPh 2 ) 2 (linear) and CH 3 C (CH 2 PPh 2 ) 3 (tripodal).
Tetradentate phosphines
An example of tetradentate tripodal phosphine ligands is tris [2- (diphenylphosphino) ethyl] phosphine (pp3).
Chiral phosphanes
There are two types of chiral phosphine ligands that play a role, for example, in asymmetric catalysis such as asymmetric hydrogenation. Chiral diphosphanes have become particularly popular. P-chiral phosphines like DIPAMP have three different substituents on phosphorus. BINAP , on the other hand, is an example of C2-symmetrical diphosphines that form chiral complexes due to atropisomerism .
| A selection of phosphine ligands | |||
| sPhos |
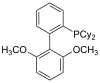
|
SPANphos |

|
| SEGphos |

|
Triphos |
|
| Xantphos |

|
Kwon phosphine |
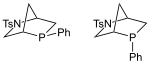
|
| Chiraphos |
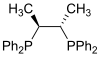
|
duPhos |
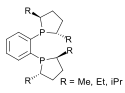
|
use
The main areas of application of phosphine ligands are a large number of homogeneous catalysis, such as the Suzuki coupling , the Buchwald-Hartwig coupling , the Sonogashira coupling or gold catalysis.
Individual evidence
- ^ JH Downing, MB Smith: Phosphorus Ligands . In: Comprehensive Coordination Chemistry II . 2003, September, pp. 253-296. doi : 10.1016 / B0-08-043748-6 / 01049-5 .
- ↑ CA Ghilardi, S. Midollini, L. Sacconi: Reactions of the tripod ligand tris (2-diphenylphosphinoethyl) phosphine with cobalt (II) and nickel (II) salts and sodium borohydride. Structural characterization of a five-coordinate cobalt (I) hydride complex . In: Inorganic Chemistry . 14, No. 8, May 2002, pp. 1790-1795. doi : 10.1021 / ic50150a010 .
- ^ Ruben Martin, Stephen L. Buchwald: Palladium-Catalyzed Suzuki - Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands . In: Accounts of Chemical Research . tape 41 , no. 11 , November 18, 2008, ISSN 0001-4842 , p. 1461–1473 , doi : 10.1021 / ar800036s , PMID 18620434 , PMC 2645945 (free full text).
- ^ Frederic Paul, Joe Patt, John F. Hartwig: Palladium-catalyzed formation of carbon-nitrogen bonds. Reaction intermediates and catalyst improvements in the hetero cross-coupling of aryl halides and tin amides . In: Journal of the American Chemical Society . tape 116 , no. June 13 , 1994, ISSN 0002-7863 , pp. 5969–5970 , doi : 10.1021 / ja00092a058 .
- ↑ Anil S. Guram, Stephen L. Buchwald: Palladium-Catalyzed Aromatic Aminations with in situ Generated Aminostannanes . In: Journal of the American Chemical Society . tape 116 , no. August 17 , 1994, ISSN 0002-7863 , pp. 7901-7902 , doi : 10.1021 / ja00096a059 .
- ↑ SYNTHESIS OF FUNCTIONALIZED ENYNES BY PALLADIUM / COPPER-CATALYZED COUPLING REACTIONS OF ACETYLENES WITH (Z) -2,3-DIBROMOPROPENOIC ACID ETHYL ESTER: (Z) -2-BROMO-5- (TRIMETHYLSILYL-4-PENTENE) ACID ETHYL ESTER . In: Organic Syntheses . tape 72 , 1995, pp. 104 , doi : 10.15227 / orgsyn.072.0104 ( orgsyn.org [accessed October 10, 2019]).
- ↑ ISOMERIZATION OF b-ALKYNYL ALLYLIC ALCOHOLS TO FURANS CATALYZED BY SILVER NITRATE ON SILICA GEL: 2-PENTYL-3-METHYL-5-HEPTYLFURAN . In: Organic Syntheses . tape 76 , 1999, pp. 263 , doi : 10.15227 / orgsyn.076.0263 ( orgsyn.org [accessed October 10, 2019]).
- ^ A. Stephen K. Hashmi: Gold-Catalyzed Organic Reactions . In: Chemical Reviews . tape 107 , no. 7 , July 2007, ISSN 0009-2665 , p. 3180-3211 , doi : 10.1021 / cr000436x .