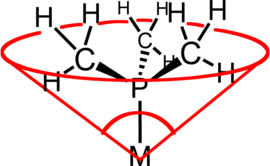
Ligand cone angle
The ligand cone angle (a common example is the Tolman cone angle or θ ) is a method of determining the steric demand of a ligand in a transition metal complex. It is defined as the solid angle that the metal forms at the apex and the extreme ends of the van der Waals radii on the circumference of the cone (see illustration). Usually tertiary phosphine ligands are classified using this parameter, but the method can be applied to any ligand. The term cone angle was first introduced by Chadwick A. Tolman , a research chemist at DuPont . He originally developed this method for phosphine ligands in nickel complexes and determined it based on measurements of accurate physical models.
Asymmetrical cases
The concept of cone angle is easiest to understand using symmetric ligands like PR 3 . However, the approach was refined to include less asymmetric ligands of the PRR'R ″ type and diphosphanes. In such asymmetrical cases, the average of half the angles, θ i / 2, between the substituents is taken to find the total cone angle θ . In the case of diphosphanes, θ i / 2 is approximately half the angle that the phosphorus atoms form with the central atom. For diphosphanes with a methylene backbone these are around 74 °, in the case of an ethylene backbone around 85 ° and in the case of a diphosphane with a propylene backbone around 90 °.
The Manz cone angle is often easier to calculate than the Tolman cone angle:
| Ligand | Angle (°) |
|---|---|
| PH 3 | 87 |
| PF 3 | 104 |
| P (OCH 3 ) 3 | 107 |
| dmpe | 107 |
| depe | 115 |
| P (CH 3 ) 3 | 118 |
| dppm | 121 |
| dppe | 125 |
| dppp | 127 |
| P (CH 2 CH 3 ) 3 | 132 |
| dcpe | 142 |
| P (C 6 H 5 ) 3 | 145 |
| P (cyclo-C 6 H 11 ) 3 | 179 |
| P ( t -Bu) 3 | 182 |
| P (C 6 F 5 ) 3 | 184 |
| P (C 6 H 4 -2-CH 3 ) 3 | 194 |
| P (2,4,6-Me 3 C 6 H 2 ) 3 | 212 |
Variations
The Tolman cone angle requires empirical binding data and defines the diameter as the maximum possible description of an idealized, freely rotating substituent. The metal – ligand distance in Tolman's model was obtained empirically from the crystal structures of tetrahedral nickel complexes. In contrast, the fixed-angle concept obtains both bond length and diameter from empirical solid-state crystal structures. Both systems have advantages.
If the geometry of a ligand is known, either through crystallography or computational calculations, an exact cone angle can be determined. In contrast to Tolman's method, no assumptions are required.
Applications
The concept of the cone angle is practically relevant in homogeneous catalysis, since the size of the ligand influences the reactivity of the attached metal center. In a known example, the selectivity of a catalytic hydroformylation is strongly influenced by the size of the coligands. And although they are monovalent , some phosphines can occupy more than half the coordination sphere of a metal center.
See also
Individual evidence
- ↑ a b c d e f g h i j k Chadwick A. Tolman: Phosphorus ligand exchange equilibriums on zerovalent nickel. Dominant role for steric effects . In: J. Am. Chem. Soc. . 92, No. 10, May 1, 1970, pp. 2956-2965. doi : 10.1021 / ja00713a007 .
- ↑ CA Tolman, WC Seidel, LW Gosser: Formation of three-coordinate nickel (0) complexes by phosphorus ligand dissociation from NiL 4 . In: J. Am. Chem. Soc. . 96, No. 1, Jan. 1, 1974, pp. 53-60. doi : 10.1021 / ja00808a009 .
- ↑ Tolman, CA: Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis . In: Chem Rev.. . 77, No. 3, 1977, pp. 313-48. doi : 10.1021 / cr60307a002 .
- ↑ TA Manz, K. Phomphrai, G. Medvedev, BB Krishnamurthy, S. Sharma, J. Haq, KA Novstrup, KT Thomson, WN Delgass, JM Caruthers, MM Abu-Omar: Structure − Activity Correlation in Titanium Single-Site Olefin Polymerization Catalysts Containing Mixed Cyclopentadienyl / Aryloxide Ligation . In: J. Am. Chem. Soc. . 129, No. 13, 2007, pp. 3776-3777. doi : 10.1021 / ja0640849 . PMID 17348648 .
- ↑ Tobias Niksch, Helmar Görls, Wolfgang Weigand: The extension of the Solid Angle Concept to bidentate ligand . In: Eur. J. Inorg. Chem. . 2010, No. 1, 2009, pp. 95-105. doi : 10.1002 / ejic.200900825 .
- ↑ Jenna A. Bilbrey, Arianna H. Kazez, J. Locklin, Wesley D. Allen: Exact ligand cone angles . In: J. Comput. Chem. . 34, No. 14, 2013, pp. 1189-1197. doi : 10.1002 / jcc.23217 . PMID 23408559 .
- ↑ CCQC . Retrieved June 2, 2016.
- ↑ Michel Petitjean: Analytical Algorithms for Ligand Cone Angles Calculations. Application to Triphenylphosphine Palladium Complexes . In: Comptes Rendus Chimie . 18, No. 6, 2015, pp. 678-684. doi : 10.1016 / j.crci.2015.04.004 .
- ↑ D. Evans, JA Osborn, G. Wilkinson: Hydroformylation of Alkenes by Use of Rhodium Complex Catalyst . In: J. Chem. Soc. . 33, No. 21, 1968, pp. 3133-3142. doi : 10.1039 / J19680003133 .