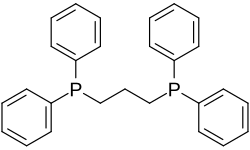
1,3-bis (diphenylphosphino) propane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,3-bis (diphenylphosphino) propane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 27 H 26 P 2 | ||||||||||||||||||
| Brief description |
colorless powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 412.46 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
60-63 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,3-bis (diphenylphosphino) propane (abbreviated: dppp ) is an organic compound . It is used as a ligand for catalytically active complexes .
presentation
Dppp can be prepared by reacting diphenylphosphine with 1,3-dibromopropane in the presence of a base . Cesium hydroxide can be used as the base for this . The base first deprotonates the phosphine , which then attacks the bromoalkane nucleophilically . Alternatively, triphenylphosphine can be reacted with lithium in THF or with sodium or potassium in ammonia to form the corresponding phenyl alkali compounds and alkali metal diphenyl phosphides and, after selective protonation of the phenyl alkali metal compounds, the remaining alkali metal diphenyl phosphide can be reacted with 1,3-dibromopropane.
use
The main area of application of dppp is its use as a bidentate chelate ligand in transition metal catalyzed coupling reactions .
The complex with nickel chloride ([Ni (dppp) Cl 2 ]) can be used as a catalyst for the alkylation of enol ethers with Grignard reagents .
Individual evidence
- ↑ a b c d e data sheet 1,3-bis (diphenylphosphino) propane (PDF) from Merck , accessed on March 17, 2011.
- ↑ MT Honaker, BJ Sandefur, JL Hargett, AL McDaniel, RN Salvatore in: Tetrahedron Lett. 2003, 44, 46, 8373-8378.
- ↑ E. Wenkert in: J. Org. Chem. 1984, 49, 4894.
