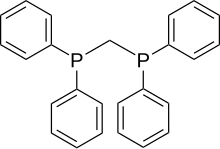
Bis (diphenylphosphino) methane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Bis (diphenylphosphino) methane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 25 H 22 P 2 | ||||||||||||||||||
| Brief description |
white crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 384.40 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
118-119 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Bis (diphenylphosphino) methane is an organic compound from the group of phosphines . It is used as a ligand for catalytically active complexes .
presentation
Dppm can be obtained from the reaction of diphenylphosphine with dichloromethane in the presence of a base . A solution of potassium hydroxide and potassium carbonate is used as the base .
Alternatively, dppm can be represented by the reaction of the less reactive triphenylphosphine with lithium and dichloromethane .
Another possibility is the reaction of chlorodiphenylphosphine with metallic lithium and dichloromethane.
use
Dppm is a common phosphine ligand in homogeneous transition metal catalysis. It usually functions as a bidentate chelating ligand , coordinating via its two phosphorus atoms on the central atom .
Individual evidence
- ↑ a b c d e f data sheet bis (diphenylphosphino) methane, 97% from AlfaAesar, accessed on December 26, 2019 ( PDF )(JavaScript required) .
- ↑ EN Tsvetkov, NA Bondarenko, IG Malakhova, MI Kabachnik: A Simple Synthesis and Some Synthetic Applications of Substituted Phosphide and Phosphinite Anions . In: Synthesis . tape 1986 , no. 3 , 1986, ISSN 0039-7881 , pp. 198-208 , doi : 10.1055 / s-1986-31510 ( thieme-connect.de ).
- ↑ Klaus Sommer: On the splitting of tertiary phosphines. I . In: Journal of Inorganic and General Chemistry . tape 376 , no. 1 , August 1970, ISSN 0044-2313 , pp. 37-43 , doi : 10.1002 / zaac.19703760106 ( wiley.com ).
- ↑ Kurt Issleib , Dietrich-Wolfgang Müller: Alkali-phosphorus compounds and their reactive behavior, III. Representation ditert. Phosphines R 2 P- [CH 2 ] n -PR 2 . In: Chemical Reports . tape 92 , no. December 12 , 1959, p. 3175-3182 , doi : 10.1002 / cber.19590921221 ( wiley.com ).
