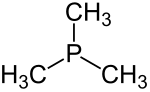
Trimethylphosphine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimethylphosphine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 9 P | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 76.08 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.738 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−86 ° C |
|||||||||||||||
| boiling point |
38-40 ° C |
|||||||||||||||
| Vapor pressure |
499 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.428 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Trimethylphosphine with the constitutional formula P (CH 3 ) 3 is an inorganic chemical compound from the group of phosphines .
Extraction and presentation
Trimethylphosphine can be obtained by reacting triphenylphosphite with methylmagnesium chloride.
properties
Trimethylphosphine is a clear, colorless liquid with a pungent odor that is insoluble in water.
use
Electron-rich phosphine ligands such as trimethylphosphine are used in numerous applications such as cross-coupling reactions. It is used as a reagent in the Mitsunobu reaction , the conversion of azides into carbamates , aziridines from azido alcohols, the production of iminophosphoranes and in the aza-Wittig reaction .
Individual evidence
- ↑ a b c d e f g h i j k data sheet Trimethylphosphine, 97% from Sigma-Aldrich , accessed on July 28, 2014 ( PDF ).
- ↑ a b Data sheet Trimethylphosphine, 99% from AlfaAesar, accessed on July 28, 2014 ( PDF )(JavaScript required) .
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2012, p. 538 ( limited preview in Google Book search).
- ↑ Optimachem: Trimethylphosphine 30% in THF
- ↑ Leutkens, Jr., ML; Sattelberger, AP; Murray, HH; Basil, JD; Fackler, Jr. JP (1990). "Trimethylphosphine". In Robert J. Angelici. Inorganic Syntheses. Inorganic Syntheses (New York: J. Wiley & Sons) 28 : 305-310. doi : 10.1002 / 9780470132593.ch76 . ISBN 0-471-52619-3 .


