Triphenyl phosphite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
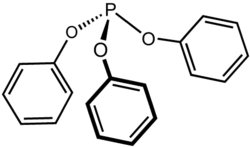
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenyl phosphite | |||||||||||||||
| other names |
Phosphorous acid triphenyl ester |
|||||||||||||||
| Molecular formula | C 18 H 15 O 3 P | |||||||||||||||
| Brief description |
colorless solid with a phenolic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 310.29 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.19 g cm −3 |
|||||||||||||||
| Melting point |
22-25 ° C |
|||||||||||||||
| boiling point |
360 ° C (1013 mbar, decomposition) |
|||||||||||||||
| Vapor pressure |
<1 mbar (20 ° C) |
|||||||||||||||
| solubility |
slow decomposition in water |
|||||||||||||||
| Refractive index |
1.59 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Triphenyl phosphite is a chemical compound from the group of phosphonic acid esters ( esters of phosphorous acid) .
Extraction and presentation
Triphenyl phosphite can be obtained by reacting phosphorus trichloride with phenol .
properties
Triphenyl phosphite is a colorless, flammable solid with a phenolic odor that slowly decomposes in water. Its aqueous solution reacts strongly acidic. He also decomposes when heated, said oxides of phosphorus , phosphine , carbon monoxide and carbon dioxide formed.
With methyl magnesium chloride it forms trimethyl phosphine .
use
Triphenyl phosphite is used as an antioxidant and complexing agent for synthetic resins , rubber and technical oils and fats.
Risk assessment
In 2013, triphenyl phosphite was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of triphenyl phosphite were concerns regarding consumer use , high (aggregated) tonnage and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances, the suspected dangers of sensitizing properties as a potential endocrine disruptor . The reassessment took place from 2013 and was carried out by the United Kingdom . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k Entry on triphenyl phosphite in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Data sheet triphenyl phosphite from Sigma-Aldrich , accessed on January 1, 2011 ( PDF ).
- ↑ Entry on triphenyl phosphite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Triphenyl phosphite , accessed on May 1, 2020.



