Phosphorus (III) fluoride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
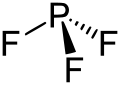
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phosphorus (III) fluoride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | PF 3 | ||||||||||||||||||
| Brief description |
colorless gas with a pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 87.97 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
3.96 g l −1 (0 ° C) |
||||||||||||||||||
| Melting point |
−152 ° C |
||||||||||||||||||
| boiling point |
−95.2 ° C |
||||||||||||||||||
| Vapor pressure |
6.9 M Pa (20 ° C) |
||||||||||||||||||
| solubility |
hydrolysis with water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
not fixed |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phosphorus (III) fluoride is a poisonous, colorless gas that is odorless in low concentrations. It hydrolyzes slowly in water and rapidly in alkalis to phosphonic acid or phosphonates . It forms stable complexes with many transition metals . In its properties as a ligand, it is similar to carbon monoxide .
Extraction and presentation
Phosphorus trifluoride is usually produced by halogen exchange from phosphorus trichloride using hydrogen fluoride , arsenic (III) fluoride , calcium fluoride or zinc fluoride .
properties
Physical Properties
The molecule has a trigonal-pyramidal structure and a PF bond angle of 96 °. The phosphorus-fluorine bond is 156 pm long, the bond enthalpy is 499 kJ / mol under standard conditions. Complex-bound phosphorus (III) fluoride shows symmetrical and asymmetrical PF stretching vibrations at a wave number of around 800 cm −1 and PF tilting vibrations at around 500 cm −1 in the infrared spectrum . In nuclear magnetic resonance spectroscopy, the phosphorus atom gives a signal at a chemical shift of 97 ppm.
| thermodynamic properties | |
| property | value |
|---|---|
| Δ f H 0 g | −919 kJ / mol |
| Δ f G 0 g | −898 kJ / mol |
| S 0 g | 273 J / (mol K) |
| C p 0 g | 59 J / (mol K) |
| p | 3.4 · 10 −30 C · m |
Chemical properties
Phosphorus (III) fluoride hydrolyzes slowly in water and quickly in alkalis to phosphonic acid and hydrofluoric acid or phosphonates and fluorides . In comparison to phosphorus trichloride, phosphorus trifluoride hydrolyzes more slowly. Phosphorus trifluoride is a very weak Lewis acid but a very strong Lewis base . As such, it forms stable complexes with many Lewis acids.
Phosphorus (III) fluoride is a weak σ donor, but a strong π acceptor. In the case of many metal carbonyls , it is able to partially or completely displace and replace the carbon monoxide ligand. It also forms complexes such as Pd (PF 3 ) 4 , the metal carbonyl analog of which is unknown. It reacts with nickel metal to form Ni (PF 3 ) 4 .
use
Phosphorus trifluoride is not used on a large scale, but in research it is used in organic synthesis and for the production of complexes.
Biological importance
Phosphorus (III) fluoride is highly toxic to humans because, like carbon monoxide, it binds strongly to hemoglobin and thus prevents oxygen breathing .
literature
- CRC Handbook of Chemistry and Physics . 60th edition. CRC Press, 1980.
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- GH Aylward, TJV Findlay: Chemistry data collection in SI units . 3. Edition. Wiley-VCH, 1999.
Individual evidence
- ↑ a b c d e f g h Entry on phosphorus trifluoride in the GESTIS substance database of the IFA , accessed on July 23, 2016 (JavaScript required)
- ^ AA Williams: Phosphorous (III) fluoride . In: Therald Moeller (Ed.): Inorganic Syntheses . tape 5 . McGraw-Hill, Inc., 1957, pp. 95-97 (English).
- ↑ G. Blyholder, R. Sheets: Metal surface interaction with Pi-acceptor molecules: PF3 adsorption. In: Journal of Colloid and Interface Science. 46, 1974, pp. 380-387, doi: 10.1016 / 0021-9797 (74) 90047-2 .
- ^ Mariusz P. Mitoraj, Artur Michalak: σ-Donor and π-Acceptor Properties of Phosphorus Ligands: An Insight from the Natural Orbitals for Chemical Valence. In: Inorganic Chemistry. 49, 2010, pp. 578-582, doi: 10.1021 / ic901736n .
- ↑ Ronald J. Clark: Phosphorus Trifluoride Substitution Compounds of Iron Pentacarbonyl. In: Inorganic Chemistry. 3, 1964, pp. 1395-1398, doi: 10.1021 / ic50020a011 .
- ↑ Geoffrey Wilkinson: The Preparation and Properties of Tetrakistribromophosphine Nickel and Tetrakistrifluorophosphine Nickel. In: Journal of the American Chemical Society. 73, 1951, pp. 5501-5502, doi: 10.1021 / ja01155a566 .





