Phosphorus (II) fluoride
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
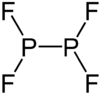
|
||||||||||
| General | ||||||||||
| Surname | Phosphorus (II) fluoride | |||||||||
| other names |
|
|||||||||
| Molecular formula | P 2 F 4 | |||||||||
| Brief description |
colorless gas |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 137.94 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| Melting point |
−86.5 ° C |
|||||||||
| boiling point |
−6.2 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Phosphorus (II) fluoride is a chemical compound with the empirical formula P 2 F 4 . The two phosphorus atoms in the +2 oxidation state are connected to one another via a single bond . Each phosphorus atom also has two bonds to fluorine atoms.
presentation
Phosphorus (II) fluoride can be produced by the reaction of phosphorodifluoride iodide with elemental mercury . Mercury (I) iodide precipitates here.
use
The compound can be used for the synthesis of difluorophosphines. So added phosphorus (II) fluoride in a gas phase reaction by UV irradiation to the triple bond of alkynes .
Individual evidence
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ JG Morse, JJ Mielcarek: Photoreactions of tetrafluorodiphosphine with alkynes. In: Journal of Fluorine Chemistry . 1988, No. 40, pp. 41-49. ISSN 0022-1139 .

