Homogeneous catalysis
Of a homogeneous catalysis is used when in a chemical reaction of the catalyst and the reactant in the same phase are present. The term is mainly used in technical chemistry to distinguish it from heterogeneous catalysis .
Acid / base-catalyzed reactions such as esterifications are homogeneous catalysis. The advantages of homogeneous catalysis over heterogeneous catalysis are the milder reaction conditions such as low pressure and moderate temperatures and the often better selectivity . However, it is disadvantageous that the catalyst is difficult to separate from the reaction mixture, since both are in the same phase.
History of homogeneous catalysis
The first catalytic technical processes used by humans are alcohol fermentation from sugar, used by the Sumerians in Mesopotamia as early as 6000 BC, as well as the production of acetic acid from alcohol with the help of catalytically active enzymes.
After these early beginnings, it was not until the 18th and early 19th centuries that a whole series of new catalytic reactions were discovered. In 1781 , Antoine-Augustin Parmentier discovered the splitting of starch into sugar under acid catalysis. Only one year later, Carl Wilhelm Scheele discovered the acid-catalyzed esterification of alcohols and acids to esters in 1782 and shortly afterwards Joseph Priestley in 1783 discovered the decomposition of ethanol into ethylene and water on alumina .
As the first process for the technical production of a basic chemical, Desormes and Clement developed the lead-chamber process for the production of sulfuric acid in 1806 , in which nitrogen oxides catalyze the oxidation of sulfur dioxide.
The French chemist Victor Henri was working in the field of enzyme catalysis as early as 1903. He investigated the breakdown of sucrose into glucose and fructose with the help of the enzyme sucrose . The continuation of his work by the German biochemist Leonor Michaelis and the Canadian physician Maud Menten succeeded in formulating the Michaelis-Menten theory in 1913 , the cornerstone of enzyme kinetics that is still valid today . The potential of enzyme catalysis for the resource-saving production of fine chemicals, pharmaceuticals, vitamins or detergents is far from being exhausted to this day, over 100 years after the fundamentals were discovered. The understanding of enzyme catalysis and its stereochemistry was expanded through the work of Cornforth , who was awarded the Nobel Prize in Chemistry for this. In addition to the development of catalytic processes for basic and intermediate products, numerous processes for the production of fine chemicals have been developed over the years.
In 1938 Otto Roelen discovered hydroformylation, the production of aldehydes from olefins, carbon monoxide and hydrogen over cobalt catalysts, which he developed further into an industrial process. Hydroformylation is considered to be the first large-scale application of homogeneous transition metal catalysts. The original Roelens process has been further developed many times. The low-pressure process developed by Karl Ziegler at the Max Planck Institute for Coal Research , in which ethylene and propylene are converted to polyolefins on titanium / aluminum catalysts, laid the foundation for the petrochemical industrial mass production of polymers that heralded the age of plastics . Ziegler and Giulio Natta were awarded the Nobel Prize in Chemistry for this work . At the MPI in Mülheim an der Ruhr, the fundamental work of Günther Wilke , who discovered the production of 1,5-cyclooctadiene from 1,3-butadiene on nickel catalysts, as well as the work of Wilhelm Keim on the SHOP process was carried out . The work of William S. Knowles and Ryoji Noyori “for their work on chirally catalyzing hydrogenation reactions” and the epoxidation named after Barry Sharpless were awarded the Nobel Prize. Also in the 1970s, Richard F. Heck discovered the cross-coupling catalyzed with homogeneous palladium complexes, which allows the direct olefination of aryl halides. Another Nobel Prize in the field of catalysis was awarded to Chauvin , Schrock, and Grubbs in 2005 for the discovery of alkene metathesis of olefins over ruthenium catalysts .
Forms of homogeneous catalysis
Acid-base catalysis
Brönsted acid / base catalysis
In Brönsted acid / base catalysis, the proton H + or the hydroxide ion OH - act as a catalyst. The substrate is activated by protonation / deprotonation. Typical Brönsted acid / base catalyzed reactions are esterifications , transesterifications or aldol reactions .
Lewis acid / base catalysis
In Lewis acid / base catalysis, the catalysts are mostly metal ions such as the titanium cation Ti 4+ or the tin cation Sn 4+ , which are often used in the form of their organic salts, such as alcoholates . Typical reactions are, for example, esterifications.
Metal complex catalysis
Redox catalysis
In redox catalysis, metal complexes act as catalysts, which catalyze the exchange of electrons between an oxidizing agent and a reducing agent. The catalyst activates the substrate through electron transfer.
Complex catalysis
In complex catalysis, metal complexes act as catalysts. The activation of the substrate takes place through coordinative interactions.
Organometallic complex catalysis
Transition metals are often used in homogeneous catalysis . The central metal atom is complexed by ligands , which means that the catalyst can have a significant influence on the selectivity and conversion of a reaction.
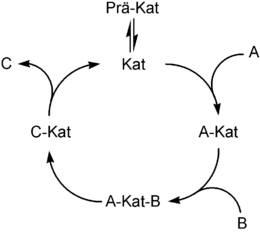
The elementary steps of homogeneous catalysis are:
- Formation of the active catalyst species (pre-cat → cat)
- oxidative addition of a substrate to the catalyst (A + Kat → A-Kat)
- Complex formation of a substrate with the catalyst (A-Kat + B → A-Kat-B)
- Insertion of a substrate into a substrate-catalyst bond (A-Kat-B → C-Kat)
- possibly a rearrangement
- reductive elimination of the product with release of the catalyst and the product (C-Kat → C + Kat)
In addition to rearrangement and insertion, oxidative addition and reductive elimination are the two most important elementary steps in homogeneous transition metal catalysis. In oxidative addition , a metal takes two previously covalently linked groups into its coordination sphere. This increases its oxidation number by two units. The starting complex must have two free coordination points for this.
The reductive elimination is the reverse of oxidative addition. Here two ligands (A and B) are removed from the coordination sphere as molecule AB and the oxidation number of the central metal atom is reduced by two.
In order to achieve a better separation, the homogeneous transition metal complex is heterogenized in some processes by using special, mostly water-soluble ligands and converted into a water phase.
In a process variant of hydroformylation, the Ruhrchemie-Rhone-Poulenc process , the catalyst is separated off using a rhodium-triphenylphosphine trisulfonate complex (TPPTS). Due to the sulfonated ligand, the catalyst remains in the aqueous phase, while the product of the process, the n- butanal produced from propene, hydrogen and carbon monoxide, forms an organic phase.
In 1966, Nozaki succeeded in using a chiral copper complex to create the first asymmetric linkage of styrene and ethyl diazoacetate to form cis - and trans-cyclopropanecarboxylates in low enantiomeric excess . In the following years the influence of chiral ligands on the stereochemical control of catalytic reactions was intensively investigated. In 2001 Barry Sharpless , Ryoji Noyori and William S. Knowles were awarded the Nobel Prize for their research in the field of asymmetric catalysis.
Organocatalysis
Small organic molecules act as catalysts in organocatalysis. The substrate is usually activated by the formation of an intermediate molecule. Both organic and inorganic substances can be obtained. A well-known example is the anthraquinone process , in which hydrogen peroxide is produced.
Enzyme catalysis
In enzyme catalysis, complex enzymes act as catalysts. The substrate activation mechanisms are diverse and range from protonation reactions to redox catalysis, for example in the case of metalloenzymes .
Catalysis by small inorganic molecules
Small inorganic molecules can act as a catalyst. In the lead chamber process , one of the first large-scale applications of homogeneous catalysis, nitrogen dioxide acts as a catalyst. In the OMEGA process, a mixture of potassium iodide / potassium molybdate has a catalytic effect both on the carbonation of ethylene oxide and on the hydrolysis of the ethylene carbonate formed. In the benzoin addition , aromatic aldehydes are added under cyanide catalysis to give aromatic α-hydroxy ketones.
solvent
The choice of solvent depends on the polarity of the substrates. Polar substrates are typically converted into alcohols or water, non-polar substrates into alkanes, or simple aromatics such as benzene or toluene. Ethers, for example tetrahydrofuran , are also used.
In addition to its dissolving properties, the solvent can act as a ligand and occupy catalytically active centers in the complex.
Technical processes
A large-scale, homogeneous catalytic process is the hydroformylation (oxo synthesis, Roelen reaction) of olefins to aldehydes with carbon monoxide and hydrogen, e.g. B. from propene to n / iso - butanal . A rhodium-triphenylphosphine complex is used here for the selective conversion of propene to n- butanal.
The Reppe reaction can be used to produce acrylic acid from acetylene , carbon monoxide and water with nickel carbonyl catalysts . Correspondingly, propionic acid can be synthesized from ethene .
Reactions catalyzed with Lewis acids allow alkylation reactions and syntheses of carboxylic acids from olefins, carbon monoxide and water or alcohols.
In the Hoechst-Wacker process, acetaldehyde is produced from ethylene and air or from pure oxygen with soluble palladium complex salts .
In hydrocyanation , the addition of HCN to an alkene is catalyzed by a nickel complex. An important synthesis is the hydrocyanation of 1,3-butadiene to adipic acid dinitrile ( DuPont ).
According to the Ziegler-Natta process , ethene is polymerized to polyethylene on an industrial scale . Accordingly, dienes can be oligomerized , e.g. B. 1,3-butadiene with nickel complexes to form cyclododeca-1,5,9-triene or to cycloocta-1,5-diene .
literature
- J. Falbe, H. Bahrmann: Homogeneous Catalysis in Technology . In: Chemistry in Our Time. 15, No. 2, 1981, pp. 37-45. doi: 10.1002 / ciuz.19810150203
- Michael Röper: Homogeneous Catalysis in the Chemical Industry . In: Chemistry in Our Time . 40, No. 2, 2006, pp. 126-135. doi: 10.1002 / ciuz.200600373
- Arno Behr: Applied Homogeneous Catalysis . Wiley-VCH, Weinheim 2008, ISBN 978-3-527-31666-3 .
Web links
Individual evidence
- ↑ a b c d e f Dirk Steinborn: Fundamentals of organometallic complex catalysis . Teubner, 2007, ISBN 978-3-8351-0088-6 ( limited preview in Google book search).
- ↑ Hubert Rehm, Friederike Hammar: Biochemistry light . Harri Deutsch Verlag, 2008, ISBN 978-3-8171-1819-9 , pp. 23 ( limited preview in Google Book search).
- ^ Boy Cornils, Wolfgang A. Herrmann, Manfred Rasch: Otto Roelen as a pioneer of industrial homogeneous catalysis . In: Angewandte Chemie . tape 106 , no. 21 , 1994, p. 2219-2238 , doi : 10.1002 / anie.19941062104 .
- ↑ Dieter Vogt: Nonaqueous Organic / Organic Separation (SHOP Process) . In: Boy Cornils, Wolfgang A. Herrmann (Ed.): Aqueous-Phase Organometallic Catalysis . John Wiley & Sons, 2004, ISBN 3-527-30712-5 , pp. 639 ff . ( limited preview in Google Book search).
- ↑ Applied homogeneous catalysis, by Arno Behr . books.google.de. Retrieved November 26, 2009.
- ↑ O. Wentzki: On the theory of the lead chamber process. In: Journal for Applied Chemistry. 23, 1910, pp. 1707-1714, doi: 10.1002 / ange.19100233603 .
- ↑ Kazuki Kawabe: Development of Highly Selective Process for Mono-Ethylene Glycol Production from Ethylene Oxide via Ethylene Carbonate Using Phosphonium Salt Catalyst. In: Catalysis Surveys from Asia. 14, 2010, pp. 111-115, doi: 10.1007 / s10563-010-9094-4 .