Organocatalysis
In organic chemistry, organocatalysis is the catalysis of organic reactions with the help of small, metal-free organic molecules that are made up of the elements carbon , hydrogen , oxygen , nitrogen , sulfur and phosphorus . The term was coined by the German chemist Wolfgang Langenbeck .
history
The beginning of organocatalysis is the benzoin addition discovered by Justus von Liebig and Friedrich Wöhler in 1832 under cyanide catalysis to form aromatic α-hydroxy ketones (benzoin).
In 1859, Justus von Liebig also discovered the oxamide synthesis from dicyan and water in the presence of acetaldehyde . Liebig identified acetaldehyde as a catalyst for the reaction and recognized its effect in parallels to the ferments ( enzymes ).
The first asymmetric organocatalytic reaction was published by Bredig and Fiske in 1912. The cyanohydrin reaction with benzaldehyde to form mandelic acid nitriles was catalyzed with alkaloids . The enantiomeric excesses achieved were around 10%.
Decades later, remarkable stereoselectivities could be achieved in an organocatalytic reaction for the first time . The amino acid proline [( S ) - or ( R ) -proline] was used as a catalyst in a Robinson annulation , which leads to the Wieland-Miescher ketone . This reaction is called the Hajos-Parrish-Eder-Sauer-Wiechert reaction after its discoverers and was of great importance for the total synthesis of steroids .
The Houk model proposed for the first time a conclusive mechanism for the metal-free enamine-aldol reaction analogous to the Zimmerman-Traxler model . Crossed direct aldol reactions were developed independently by List, Barbas, Shibasaki, and Trost . The first organocatalytic cross-aldol reaction of aldehydes was reported by MacMillan in 2002.
Reaction mechanism
The catalyst can be covalently bonded to the substrate molecule in the catalytic cycle; in this case, relatively high concentrations of the organocatalyst are required. Catalytic, non-covalent interactions, for example via hydrogen bonds, are also possible, which only require small amounts of the catalyst.
Covalent Mechanism
The principle of most organocatalytic processes is that the catalyst first reacts with a reaction partner to form a (reversible) covalent bond. In the proline-catalytic aldol reaction, ( S ) -proline first condenses on the ketone used . The resulting iminium ion then tautomerizes to the enamine , which in the next step nucleophilically attacks the aldehyde used . Subsequent hydrolysis releases the product and reforms ( S ) -proline.
In the reaction, the stereo information is transmitted through the (chiral) ( S ) -proline. The carboxy group of ( S ) -proline also activates the aldehyde by forming a hydrogen bond . The reaction proceeds via a six-membered chair-like transition state similar to the Zimmerman-Traxler model for lithium enolates. The aldehyde substituent is in the pseudo-equatorial plane.
The course of the reaction via a chair-like transition state was first postulated by quantum chemical calculations by Houk and later proved experimentally by List by oxygen labeling. If the non-proteinogenic amino acid ( R ) -proline is used instead of ( S ) -proline , the enantiomeric aldol is formed with the same stereoselectivity.
Non-covalent organocatalysis
In the case of non-covalent organocatalysis, no covalent bonds are formed with the catalyst. It is based on weak, directional interactions between an organocatalyst and the substrate to be activated. Many enzymes , which also serve as models for the development of non-covalent organocatalysts, also react according to this principle . Derivatives of urea or thiourea , for example, are used as neutral hydrogen bond donors . Catalysts which have proven to be favorable here are those which are poor in electrons and have a rigid structure and have a phenyl ring which carries electron-withdrawing, non-coordinating substituents in the 3,4 and / or 5-position .
Advantages of thiourea derivatives (especially compared to traditional, metal-containing Lewis acid catalysts):
- non-covalent bond to the substrate and thus low product inhibition
- low catalyst loading (up to 0.001 mol%), high TOF values
- simple and cheap synthesis and structural modification
- Connection to the solid phase; thus recovery possible
- not sensitive to air or water, no inert gas atmosphere required, unproblematic handling
- enable catalysis under almost neutral conditions, tolerance of acid-labile substrates
- metal-free, non-toxic like many metal-containing Lewis acid catalysts
- environmentally friendly (" green chemistry ")
Reactions
There are already effective organocatalysts for the following reactions:
- Aldol reactions (e.g. Hajos-Parrish-Eder-Sauer-Wiechert reaction )
- Michael additions
- Mannich reactions
- α- aminations
- Diels-Alder and hetero- Diels-Alder reactions
- Knoevenagel condensations
- Wittig rearrangements
- Fluorinations
- Reductions
- asymmetric Stetter reactions
- Nitroaldol reaction
- Shi epoxidation
- Baylis-Hillman reaction
- Ketone reduction
Catalysts derived from natural products
Catalysts derived from the amino acids ( S ) - proline were and are frequently used. Catalysts derived from ( S ) - phenylalanine are also often used.
Some of the catalysts used in organocatalytic reactions are derived from the Cinchona (China) alkaloids :
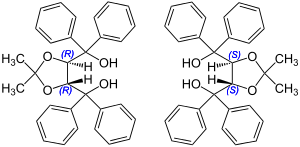
Catalysts derived from tartaric acid , for example TADDOL E, are also used in organocatalytic reactions:
MacMillan catalysts
The first catalysts for enantioselective organocatalytic Diels-Alder reactions were developed by MacMillan:
Individual evidence
- ↑ W. Langenbeck: The organic catalysts and their relationships to the ferments , Springer, Berlin 1949.
- ↑ F. Wöhler and J. Liebig, Ann. Pharm. 1832, 3, 249.
- ↑ J. v. Liebig : About the formation of the oxamide from cyan , in: Liebigs Ann. 1860 , 113 , 246-247; doi : 10.1002 / jlac.18601130213 .
- ↑ G. Bredig , PS Fiske, Biochem Z 1912 , 46 , 7.
- ↑ U. Eder, G. Sauer, R. Wiechert: Novel asymmetric cyclization to optically active steroid CD fragments , in: Angew. Chem. 1971 , 10 , 492-493; doi : 10.1002 / anie.19710831307 .
- ↑ ZG Hajos, DR Parrish: Asymmetric Synthesis of Optically Active Polycyclic Organic Compunds. German patent DE 2102623, July 29, 1971.
- ↑ ZG Hajos, DR Parrish: Asymmetric synthesis of bicyclic intermediates of natural product chemistry , in: J. Org. Chem. 1974 , 39 , 1615; doi : 10.1021 / jo00925a003 .
-
↑ a b K. N. Houk, S. Bahmanyar: The Origin of Stereoselectivity in Proline-Catalyzed Intramolecular Aldol Reactions. In: J. Am. Chem. Soc. 2001, 123, 12911-12912, doi : 10.1021 / ja011714s .
S. Bahmanyar, KN Houk: Transition States of Amine-Catalyzed Aldol Reactions Involving Enamine Intermediates: Theoretical Studies of Mechanism, Reactivity, and Stereoselectivity. In: J. Am. Chem. Soc. 2001, 123, 11273-11283, doi : 10.1021 / ja011403h . - ^ B. List, RA Lerner, CF Barbas, III., Proline-Catalyzed Direct Asymmetric Aldol Reactions in: J. Am. Chem. Soc. 2000 , 122 , 2395-2396, doi : 10.1021 / ja994280y .
- ↑ S. Kandasamy, W. Notz, T. Bui, CF Barbas, III., Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon − Carbon Bond-Forming Reactions in: J. Am. Chem. Soc. 2001 , 123 , 5260-5267, doi : 10.1021 / ja010037z .
- ↑ YMA Yamada, N. Yoshikawa, H. Sasai, M. Shibasaki: Direct catalytic asymmetric aldol reactions of aldehydes with unmodified ketones in: Angew. Chem. 1997 , 109 , 1842-1944; doi : 10.1002 / anie.19971091716 .
- ↑ BM Trost, H. Ito, A Direct Catalytic Enantioselective Aldol Reaction via a Novel Catalyst Design in: J. Am. Chem. Soc. 2000 , 122 , 12003-12004, doi : 10.1021 / ja003033n .
- ^ AB Northrup, DWC MacMillan, The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes in: J. Am. Chem. Soc. 2002 , 124 , 6798-6799, doi : 10.1021 / ja0262378 .
- ↑ Linh Hoang, KN Houk, S. Bahmanyar, B. List: Kinetic and Stereochemical Evidence for the Involvement of Only One Proline Molecule in the Transition States of Proline-Catalyzed Intra- and Intermolecular Aldol Reactions. In: J. Am. Chem. Soc. 2003, 125, 16-17.
- ↑ MS Taylor, EN Jacobsen: Asymmetric Catalysis by Chiral Hydrogen-Bond Donors , in: Angew. Chem. Int. Ed. 2006 , 45 , 1520-1543; doi : 10.1002 / anie.200503132 .
- ↑ SJ Connon: Organocatalysis Mediated by (Thio) urea Derivatives , in: Chem. Eur. J. 2006 , 12 , 5418-5427; doi : 10.1002 / chem.200501076 .
- ^ D. Seebach , AK Beck, DM Badine, M. Limbach, A. Eschenmoser , AM Treasurywala, R. Hobi, W. Prikoszovich, B. Linder: Are Oxazolidinones really unproductive, parasitic species in proline catalysis? Thoughts and experiments pointing to an alternative view , in: Helv. Chim. Acta 2007 , 90 , 425-471.
- ↑ S. Mukherjee, JW Yang, S. Hoffmann, B. List: Asymmetric enamine catalysis , in: Chem. Rev. 2007 , 107 , 5471-5569.
- ↑ KA Ahrendt, CJ Borths, DWC MacMillan, New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels-Alder Reaction in: J. Am. Chem. Soc. 2000 , 122 , 4243-4244, doi : 10.1021 / ja000092s .
- ^ NA Paras, DWC MacMillan, New Strategies in Organic Catalysis: The First Enantioselective Organocatalytic Friedel-Crafts Alkylation in: J. Am. Chem. Soc. 2001 , 123 , 4370-4371, doi : 10.1021 / ja015717g .
- ^ AB Northrup, DWC MacMillan, The First General Enantioselective Catalytic Diels − Alder Reaction with Simple α, β-Unsaturated Ketones in: J. Am. Chem. Soc. 2002 , 124 , 2458-2460, doi : 10.1021 / ja017641u .
literature
- MS Taylor, EN Jacobsen: Asymmetric Catalysis by Chiral Hydrogen-Bond Donors , in: Angew. Chem. Int. Ed. 2006 , 45 , 1520-1543; doi : 10.1002 / anie.200503132 .
- SJ Connon: Organocatalysis Mediated by (Thio) urea Derivatives , in: Chem. Eur. J. 2006 , 12 , 5418-5427; doi : 10.1002 / chem.200501076 .
- B. List (Ed.): Organocatalysis , Thematic Series (Open Access) in the Beilstein Journal of Organic Chemistry.
Web links
- Information sources, data, description, background information on the subject of organocatalysis
- A. Berkessel, H. Gröger : Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis , Wiley-VCH, Weinheim 2005. ISBN 978-3-527-30517-9