Robinson annulation
The Robinson annulation is a name reaction of organic chemistry and named after the British chemist and winner of the Nobel Prize for Chemistry Robert Robinson . The reaction makes it possible to convert ketones and α, β-unsaturated ketones in a basic medium. A six-membered ring is formed and organic compounds can be expanded to include a six-membered ring. Other ring sizes are not possible. The Robinson annulation consists of two decisive reaction steps: a Michael addition and the subsequent aldol condensation .
Reaction mechanism
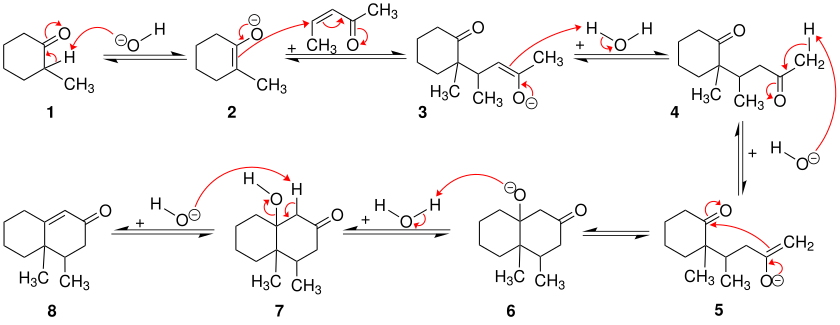
The Robinson annulation is explained in this section using a cyclohexanone derivative and an unsaturated pentanone.
The first steps of the Robinson annulation are the same as the Michael addition . The cyclohexanone derivative 1 is first deprotonated by a base in the α-position to the carbonyl group. This creates enolate 2 , which nucleophilically attacks the α, β-unsaturated ketone. After protonation, the 1,5- diketone 4 and again the base are formed, which shows its role as a catalyst. The further steps belong to an aldol reaction . The 1,5-diketone 4 is deprotonated again by the base in the α-position. A ring closure then takes place in that the enolate 5 attacks the other carbonyl group in the molecule. After protonation of the alcoholate 6 , the β-hydroxyketone 7 ( aldol ) is formed, which gives the α, β-unsaturated ketone 8 with elimination of water .
When the reaction is complete, an unsaturated carbonyl compound is formed again , which, as a so-called Michael acceptor, can serve as a starting point for another Robinson annulation. This method is particularly suitable for building up steroid structures .
variants
The Wieland-Miescher ketone is racemic per se. If, however, the Robinson annulation is catalyzed with the help of L - or D - proline - i.e. a chiral enantiomerically pure organic catalyst - the ( R ) - is formed in an enantioselective reaction (called Hajos-Parrish-Eder-Sauer-Wiechert reaction ) or ( S ) -enantiomer of the Wieland-Miescher ketone is preferred.
literature
- Bergmann, ED; Gingberg, D .; Pappo, R. Org. React. 1959 , 10 , 179.
Individual evidence
- ↑ Rapson, William Sage; Robinson, Robert: In 307. Experiments on the synthesis of substances related to the sterols. Part II. A new general method for the synthesis of substituted cyclohexenones , Journal of the Chemical Society 1935 , 1285 doi : 10.1039 / JR9350001285 .
- ↑ KPC Vollhardt, NE Schore, Organic Chemistry, 4th Edition, Wiley-VCH Verlag, 2005, p. 944, ISBN 3-527-31380-X .
- ^ Organikum, 16th edition, VEB Deutscher Verlag der Wissenschaften Berlin 1985, p. 511, ISBN 3-326-00076-6 .
- ↑ Wieland, P .; Miescher, K .: In About the Production of Polynuclear Ketones. , Helv. Chim. Acta 1950 , 33 , 2215. doi : 10.1002 / hlca.19500330730 .
- ↑ Hajos, Zoltan G., Parrish, David R. German Offenlegungsschrift 2102623 (January 21, 1970).