Hajos-Parrish-Eder-Sauer-Wiechert reaction
The Hajos-Parrish-Eder-Sauer-Wiechert reaction is a reaction in organic chemistry . It is a proline -catalyzed asymmetric aldol reaction . The reaction was named after the discoverers at Hoffmann-La Roche and Schering AG .
history
The original process was discovered by Zoltan Hajos and David R. Parrish in the 1970s and yields the optically active aldol product - a bicyclic ketol. The Eder-Sauer- Wiechert modification adds a condensation step to the reaction and the optically active endion is obtained via this asymmetric organocatalytic reaction. The reaction and the resulting chiral compounds were common in steroid use synthesis to the C- and D-ring of the Steran build -Gerüstes. The reaction had often been used in the synthesis of other optically pure molecules.
In the original work, naturally occurring proline is used as a chiral catalyst for an aldol reaction. The starting compound is an achiral triketone and only 3 mol% ( S ) -proline are used to produce a ketol with 93% enantiomeric excess . They worked at moderate temperatures and DMF as the solvent . In contrast, the Schering group worked under non-biological conditions with 47 mol% ( S ) -proline in a 1N perchloric acid and acetonitrile as a solvent at 80 ° C. The product found by Hajos and Parrish was therefore not isolated, but instead the unsaturated product of the aldol condensation.
Hajos and Parrish determined the absolute configuration for the cis -linked 7a-methyl-6,5-bicylic ketol by CD spectroscopy and confirmed these results with the aid of X-ray structure analysis. The structure of the ketol is therefore as follows:
In 2000, a study by Benjamin List et al. showed that an aldol addition between a ketone and an aldehyde in the presence of ( S ) -proline also leads to an asymmetric induction with a clear enantiomeric excess.
The authors explicitly pointed out the similarity between the proline-catalyzed asymmetric aldol reaction and the enzyme aldolase A , because both have an enamine intermediate as a transition state.
Reaction mechanism
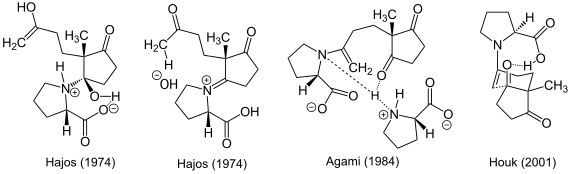
A number of proposals have been made about the mechanism of asymmetric catalysis of the reaction. Hajos proposed in 1974 a hemiaminal as a key intermediate prior, due to its experiments with a stoichiometric amount of H 2 18 O water while Agami in 1984 an enamine preferred as an intermediate. Through experimental results, he proposed an enamine with two proline molecules as a transition state based on the kinetic data . The mechanism of the transition state proposed by Kendall Houk in 2001 is corresponding . A single proline molecule is sufficient here to form the transition state with the help of a hydrogen bond .
The reaction mechanism, as presented by List's group in 2000, is based on the formation of an enamine and the observed stereochemistry can be explained with the help of the Zimmerman-Traxler model .
Several factors play a role in the stereogenic step of the reaction: The carboxy group of proline approaches the carbonyl oxygen atom from the same half-space and activates it electrophilically through a hydrogen bond . The chair-shaped transition state carries its substituents either axially (Si facial attack) or equatorially (Re facial attack). Since the transition state with equatorially positioned substituents is energetically more favorable, the reaction continues accordingly.
Origin of the name of the reaction
In 1985 C. Agami first described the proline-catalyzed asymmetric Robinson annulation as the Hajos-Parrish reaction. Even Henri Kagan called (along with Agami) this reaction in a publication with this name. Later in 2001, Kagan published a paper and referred to the reaction here as the Hajos-Parrish-Wiechert reaction. In 2002, two names were added in a publication by Benjamin List and so the reaction is now practically uniformly referred to in the scientific literature as the Hajos-Parrish-Eder-Sauer-Wiechert reaction. The original process provides the optically active aldol product and is known as the Hajos-Parrish reaction.
Individual evidence
- ↑ Patent DE2102623 : Asymmetric synthesis of polycyclic organic compounds. Published July 29, 1971 , Inventor: ZG Hajos, DR Parrish.
- ↑ Zoltan G. Hajos, David R. Parrish: Asymmetric synthesis of bicyclic intermediates of natural product chemistry. In: Journal of Organic Chemistry . Volume 39, 1974, pp. 1615-1621, doi : 10.1021 / jo00925a003 .
- ↑ Ulrich Eder, Gerhard Sauer, Rudolf Wiechert: New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. In: Angewandte Chemie . International edition in English. Volume 10, 1971, pp. 496-497, doi : 10.1002 / anie.197104961 .
- ^ Zerong Wang in Comprehensive Organic Name Reactions and Reagents. 3 volumes, pp. 1305-1309, John Wiley and Sons Inc., 2009, doi: 10.1002 / 9780470638859.conrr290 .
- ^ Benjamin List : Proline-catalyzed asymmetric reactions. In: Tetrahedron . Volume 58, 2002, pp. 5573-5590, doi : 10.1016 / S0040-4020 (02) 00516-1 .
- ^ IL Karle, J. Karle : The crystal structure of digitoxigenin. In: Acta Crystallographica . Section B, Vol. 25, 1969, pp. 434-442.
- ^ A b Benjamin List, Richard A. Lerner , Carlos F. Barbas III: Proline-Catalyzed Direct Asymmetric Aldol. In: Journal of the American Chemical Society . Volume 122, 2000, pp. 2395-2396, doi : 10.1021 / ja994280y .
- ^ Claude Agami, Franck Meynier, Catherine Puchot, Jean Guilhem and Claudine Pascard: Stereochemistry-59: New insights into the mechanism of the proline-catalyzed asymmetric robinson cyclization; structure of two intermediates. asymmetric dehydration. In: Tetrahedron. Volume 40, 1984, pp. 1031-1038, doi : 10.1016 / S0040-4020 (01) 91242-6 .
- ^ S. Bahmanyar and KN Houk: The Origin of Stereoselectivity in Proline-Catalyzed Intramolecular Aldol Reactions. In: Journal of the American Chemical Society. Volume 123, 2001, pp. 12911-12912, doi : 10.1021 / ja011714s .
- ^ S. Bahmanyar and NK Houk: Transition States of Amine-Catalyzed Aldol Reactions Involving Enamine Intermediates: Theoretical Studies of Mechanism, Reactivity, and Stereoselectivity. In: Journal of the American Chemical Society. Volume 123, 2001, pp. 11273-11283, doi : 10.1021 / ja011403h .
- ↑ Reinhard Brückner: Reaction Mechanisms, Springer Spectrum, Edition: 3rd Edition 2004, p. 507.
- ↑ C. Agami, J. Levisalles, CJ Puchot: A New Diagnostic Tool for Elucidating the Mechanism of Enantioselective Reactions. Application to the Hajos-Parrish Reaction. In: Journal of the Chemical Society, Chemical Communications . Volume 8, 1985, pp. 441-442.
- ↑ C. Puchot, O. Samuel, E. Dunach, S. Zhao, C. Agami and HB Kagan: Nonlinear Effects in Asymmetric Synthesis. In: Journal of the American Chemical Society. Volume 108, 1986, pp. 2353-2357.
- ^ HB Kagan et al: Nonlinear Effects in Asymmetric Catalysis: A Personal Account. In: Synlett . 2001, pp. 888-899.
- ↑ KN Houk, PH-Y. Cheong: Computational prediction of small molecule catalysts. In: Nature . Volume 455, 2008, pp. 309-313, doi : 10.1038 / nature07368 .
- ^ SE Wheeler, A. Moran, SN Pieniazek and KN Houk: Accurate Reaction Enthalpies and Sources of Error in DFT Thermochemistry for Aldol, Mannich, and R-aminoxylation Reactions. In: Journal of Physical Chemistry. Series A, Volume 113, 2009, pp. 10376-10384.