Steroids
Steroids ( Greek στερεοειδές , from στερεός stereós , German 'solid' and the adjective suffix -id , Latinization from ancient Greek -ειδής -eidḗs "[similar to the noun ]", from εἶδος eîdos "appearance, shape, type of lipids ") are a substance (Molecules with lipophilic groups, usually insoluble in water). Formally, the steroids are derivatives of the hydrocarbon sterane (cyclopentanoperhydrophenanthrene). Steroids belong to the isoprenoids , more precisely to the triterpenoids .
Natural steroids are found in animals, plants, and fungi ; many are synthesized in the smooth endoplasmic reticulum . Their biochemical tasks range from the production of vitamins and sex hormones ( androgens in men and estrogens in women) through bile acid and toad poisons to the cardiac toxins of digitalis and oleander .
The name of the substance class is derived from the first known steroid, cholesterol . In animals and in the human organism, cholesterol is the most important steroid; Plants, on the other hand, do not contain it. Lipoproteins and steroid hormones are built up from cholesterol , e.g. B. the hormones of the adrenal cortex ( corticosteroids ). Artificial derivatives of the steroid male sex hormone testosterone , the anabolic steroids , are used as muscle building preparations and are therefore also known as doping agents .
The total synthesis of steroids first succeeded in 1939 in equilenin and 1948 with estrone , both aromatic steroids. The breakthrough in non-aromatic steroids such as cholesterol and cortisone was achieved independently in 1951 by the groups of Robert B. Woodward in the USA and Robert Robinson in England.
structure
The basic structure of steroids is the sterane . One structural commonality is the cyclopentanoperhydrophenanthrene ring (exception: vitamin D ). Steroids have a rigid molecular shape, usually a relatively high melting point, and are easy to crystallize. As a result of the asymmetric carbon atoms on the ring links, numerous structural isomers are possible that are folded differently. Not all possible folds occur in nature. According to the general convention, the position of the methyl group at carbon 10 serves as a reference point for the systematic naming of isomers: To the methyl group trans -ständige be Substitutienten with the index α (alpha) referred to, cis -ständige with β (beta). For bile acids z. B. the rings A and B are cis -linked (90 ° angle), they belong to the 5β-androstanes. In contrast, steroid hormones are trans- linked at this point ( 5α-androstanes ). Subgroups are abbreviated (e.g. "-ol" = alcohol group). The position of double bonds is indicated with a Δ (delta). The systematic name of cholesterol is e.g. B. Cholest-Δ5-en-3β-ol.
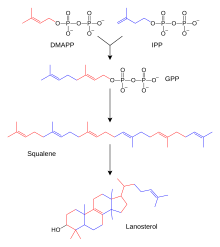
Biosynthesis of steroid hormones
The biosynthesis of steroids is basically the same as the biosynthesis of terpenes . An important intermediate step leads to squalene , a triterpene. Lanosterol is created through several cyclic linkages. This steroid with Sterangrundgerüst supplies with elimination of three methyl groups, hydrogenation and isomerization cholesterol . Aldosterone , testosterone and cortisol are formed from cholesterol in three different ways . This happens in the adrenal cortex and in the male and female gonads ( testicles and ovary ). Testosterone (male sex hormone) is first produced in the ovary, which is then converted into estradiol using an aromatase (enzyme that dehydrates ring A of the steroid structure into a benzene ring ) . The enzymes that catalyze the individual steps from cholesterol to steroid hormones can be disrupted by genetic defects. The 21-hydroxylase deficiency is relatively common . This leads to an overproduction of sex hormones, as the path to cortisol and aldosterone is disturbed. The disease is called adrenogenital syndrome .
Dismantling
In humans, the steroids are made water-soluble in the liver by hydroxylation and conjugation with glycine or taurine and excreted as bile acids via the bile into the duodenum (anterior part of the small intestine).
Classification
- Sterols (sterols) [for example cholesterol (cholesterol), ergosterol (ergosterol)]
- Bile acids ( e.g. cholic acid )
-
Steroid hormones
- Glucocorticoids (such as cortisone )
- Mineralocorticoids ( e.g. aldosterone )
- Estrogens ( e.g. estrone , estradiol )
- Progestins (such as progesterone )
- Androgens (e.g. testosterone , androsterone )
- Insect hormones ( e.g. ecdysone )
- steroids that act on the heart ( aglycones of cardiac glycosides )
- Cardenolides ( e.g. digoxigenin , digitoxigenin , gitoxigenin , strophanthidin )
- Bufadienolides (e.g. Cillarenin , hellebrigenin , uzarigenin )
- Sapogenins (e.g. digitogenin , diosgenin )
- Steroid alkaloids ( e.g. solanidine , tomatidine )
literature
- Christoph Rüchardt: The discovery and structure of steroids. Works by Heinrich Kilian (1855–1945), Adolf Windaus (1876–1959) and Heinrich Wieland (1877–1957) . In: 550 years of the Albert Ludwig University of Freiburg . Volume 4. Alber, Freiburg im Breisgau 2007, ISBN 978-3-495-48254-4 , pp. 207-210, uni-freiburg.de
Web links
- IUPAC nomenclature of steroids (English)
Individual evidence
- ↑ Entry on steroids. In: Römpp Online . Georg Thieme Verlag, accessed on April 2, 2014.
- ^ Zdzislaw ZE Sikorski: Chemical and Functional Properties of Food Lipids. CRC Press, 2010, ISBN 978-1-420-03199-7 , p. 41.
- ^ WE Bachmann , W. Cole, AL Wilds, J. Am. Chem. Soc., Vol. 61, 1939, p. 974, Vol. 62, 1940, p. 824.
- ↑ G. Anner, Karl Miescher , Experientia, Volume 4, 1948, p. 25, Helv. Chim. Acta, Volume 31, 1948, p. 2173, Volume 32, 1949, p. 1957.
- ↑ RB Woodward, F. Sondheimer, D. Taub, K. Heusler, WM McLamore, J. Am. Chem. Soc., Vol. 74, 1952, p. 4223.
- ↑ HME Caldwell, JW Cornforth, SR Duff, H. Holtermann, R. Robinson, Chem. Ind., London, 1951, p. 389, J. Chem. Soc., London, 1953, p. 361.