Adrenogenital Syndrome
| Classification according to ICD-10 | |
|---|---|
| E25.0 | Congenital adrenogenital disorders associated with enzyme deficiency |
| ICD-10 online (WHO version 2019) | |
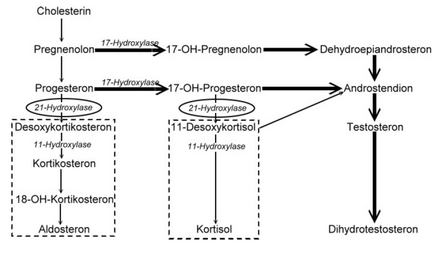
The adrenogenital syndrome ( AGS ) is a group of autosomal - recessive inherited metabolic diseases caused by a disturbance of hormone synthesis in the adrenal cortex are presented, wherein an overproduction of androgens adrenal steroid is (sex hormones of the adrenal cortex). The formation of cortisol and aldosterone is disturbed. Overstimulation of the adrenal cortex increasingly activates secondary metabolic pathways and forms precursors (e.g. pregnenolone , progesterone ). The lack of cortisol leads to the compensatory stimulation of the entire adrenal gland by the hypothalamus and pituitary gland . Since the formation of the sex hormones - which is not disturbed - also takes place in the adrenal cortex, masculinization or premature sexual development in boys occurs in girls. The lack of aldosterone leads to disturbances in the salt balance with loss of fluids. For treatment, the missing hormones must be replaced for life.
Cause and classification
Adrenogenital syndrome is divided into five types depending on the enzyme involved . The most common form, with over 90% of cases, is type 3 - also known as adrenogenital salt loss syndrome or Debré-Fibiger syndrome - with a disruption of the enzyme 21-hydroxylase . Depending on the course, a distinction is made between two forms of 21-hydroxylase deficiency. In classic AGS , the symptoms already exist in newborns. It can be associated with salt loss, if aldosterone production is also disturbed. If the latter is not affected, there is no loss of salt. The non-classic AGS manifests itself later, usually during puberty or even in adulthood, and is characterized by much milder symptoms.
| Type | affected enzyme | Locate | Frequency (births) |
| Type 1 (lipoid) | StAR protein | 8p11.2 | rare in Europe |
| Type 1 (lipoid) | Cholesterol monooxygenase | 15q23-24 | only one known case |
| Type 2 | 3beta-hydroxysteroid dehydrogenase | 1p13.1 | |
| Type 3 | 21-hydroxylase | 6p21.3 | 1: 5,000 - 15,000 |
| Type 4 | 11-beta hydroxylase | 8q24.3 | 1: 100,000 |
| Type 5 | 17alpha-hydroxylase | Rare |
Defects in the StAR protein occur more frequently in Japan and South Korea .
Pathophysiology
Since the production of the adrenal cortex hormones is subject to a control loop , the hypothalamus and the pituitary gland ( pituitary gland ) try to compensate for the cortisol deficiency by increasing the release of the adrenal cortex-stimulating hormone ACTH (adrenocorticotropic hormone). This leads to an increase in the size of the adrenal cortex ( adrenal hyperplasia ) and the synthesis cascade of the adrenal cortex hormones is strongly stimulated up to the point of the actual enzyme defect. In addition, the increased ACTH secretion can pathologically directly stimulate the synthesis of all corticoids, including androgens in particular, the secretion of which is not disturbed. There is an increased formation of the hormone precursors ( steroids ), which, due to the lack of enzymes, are broken down neither into cortisol nor aldosterone, but into androgens via alternative metabolic pathways . The androgens lead to virilization .
The salt loss is caused by the lack of mineralocorticoid activity, even if the synthesis of aldosterone is disturbed. This can occur because aldosterone synthesis relies to a large extent on enzymes identical to those used for cortisol synthesis.
Symptoms
The symptoms not only vary depending on the course, but also differ in the two sexes, as they are caused to a large extent by an overproduction of male sex hormones (androgens) .
Classic adrenogenital syndrome without salt loss
Girls who suffer from this disorder are born with a masculinized external genitalia , i.e. an almost penis-like clitoris that is enlarged to different degrees , since they were already under the influence of the increased androgens in the womb. The inner genitals are female. At birth, boys have a normal external sex organ with partially hyperpigmented scrotum . If the disease remains undetected and, accordingly, untreated, the children will be noticed by an apparent early development of puberty ( pseudopubertas praecox ). Armpit and pubic hair can already develop in childhood, the penis is greatly enlarged, but the testicles remain childlike. Hirsutism develops in girls . The growth in length is initially accelerated, so that the children are initially too big for their age. However, since there is also premature bone maturation and premature closure of the growth plates ( epiphyseal plates ), this ultimately results in short stature . Other possible symptoms include acne , lack of breast development, menstrual cycle disorders, and infertility.
Classic adrenogenital syndrome with salt loss
In this form, the children get additional problems due to the lack of mineralocorticoid effect of the insufficiently formed aldosterone. This is largely responsible for the reuptake of sodium ions from the primary urine by the kidneys . In the first days or weeks of life, affected infants develop a severe disruption of the salt balance ( hyponatremia and hyperkalaemia ) with vomiting and weight loss due to metabolic acidosis of the blood . The children become increasingly listless. This salt loss crisis can reach life-threatening proportions.
Non-classical adrenogenital syndrome
This form of the course, also known as late-onset AGS , is characterized by mild symptoms of simple AGS without loss of salt. Girls have normal genitals at birth. Premature pubic hair and acne occur in both sexes. Women get disorders of the menstrual cycle and conception. Fertility can be impaired. The short stature is only slightly noticeable in both sexes. A minimal form of AGS, in which no symptoms can be observed, but measurable changes in the hormone concentrations, is also called non-classic cryptic AGS .
diagnosis
In the event of symptoms of a salt loss crisis, a determination of the electrolytes in the serum and the acid-base status ( blood gas analysis ) can provide information on the severity of the derailment. If the symptoms suggest the presence of an adrenogenital syndrome, the diagnosis can first be confirmed by determining the hormone concentrations, especially 17-hydroxyprogesterone in the blood. As an alternative metabolic product, this is significantly increased in the case of disturbed cortisol synthesis. In Germany, the determination of 17-hydroxyprogesterone is now part of the extended newborn screening and is routinely determined in all newborns. A molecular genetic examination can prove the known underlying genetic defects. This can also be done in the unborn child in the womb by means of an amniocentesis or a chorionic villus sampling , so that affected girls can already be treated intrauterine by corticosteroid substitution . To heterozygous but can pass on the change to their children carriers, which themselves do not become ill to identify, one can ACTH test to be performed. The adrenal gland is stimulated to increase hormone production by administration of the adrenal cortex-stimulating hormone. If there is a change in only one allele of the 21-hydroxylase gene, there is an increased increase in 17-hydroxyprogesterone.
therapy
Since the cause of the disease lies in a genetic defect, a causal treatment is not possible according to the current state of science. The symptomatic therapy consists in a lifelong substitution of the missing hormones . As a result, the production of ACTH in the pituitary gland decreases again, the production of androgens decreases, the adrenal cortex shrinks back to normal size and the symptoms of hormone deficiency disappear. The therapy takes place with hydrocortisone (in adults often also prednisone or dexamethasone ) and can take the form of tablets. Since aldosterone itself is not absorbed, it is substituted with the mineralocorticoid fludrocortisone . Therapy should be started as early as possible. It must also be noted that the need for adrenal cortex hormones is significantly increased in stressful situations. This is why the dose of hydrocortisone must be doubled in the case of acute illnesses or before operations (fludrocortisone is not increased). There is no indication to interrupt the therapy. All AGS patients must be given an emergency ID card.
forecast
If the hormone substitution is well adjusted, the prognosis of the adrenogenital syndrome is extremely good. Symptoms disappear and patients can lead normal lives and achieve normal fertility .
See also
- Adrenal insufficiency (Addison's disease)
- Adrenomyodystrophy
- PCO syndrome (as a diagnosis of exclusion after diagnosis of the non-classical adrenogenital syndrome)
- Pre-puberty (as a differential diagnosis)
literature
- Herbert Stolecke (ed.): Endocrinology of childhood and adolescence . Springer, Berlin / Heidelberg / New York 1997, ISBN 3-540-61855-4 .
Web links
- AGS in "Laborlexikon" ISSN 1860-966X
- AWMF guideline "Disorders of Gender Development" with AGS
- AWMF guideline "Adrenogenital Syndrome"
- AGS - Parents and Patients Initiative V.
- AGS-Forum.de - A community for sharing life with AGS
Individual evidence
- ↑ eesom.com practical med. Sidonie Achermann, doctor
- ↑ Adrenogenital Syndrome. on: tk.de
- ↑ G. Herold: Internal Medicine. Self-published, 2002.
- ^ PP Nawroth: Clinical Endocrinology and Metabolism. Springer, 2001.
- ↑ G. Strohmeyer: Congenital metabolic diseases. Ecomed, 2002.
- ↑ DP Merke u. a .: Congenital adrenal hyperplasia. In: Lancet. 2005; 365, pp. 2125-2136.
- ↑ AWMF guideline adrenogenital syndrome , guidelines register number 027/047
- ↑ OMIM entry for type 1
- ^ A b Hans-Christian Pape, Armin Kurtz, Stefan Silbernagl : Physiology . 7th edition. Georg Thieme Verlag, Stuttgart 2014, ISBN 978-3-13-796007-2 , p. 612, 613 .
- ↑ Dietrich V. Michalk, Wiebke Ahrens, Eckhard Schönau: Differentialdiagnose Pädiatrie. Elsevier, Urban & Fischer-Verlag, 2005, ISBN 3-437-22530-8 , p. 419.
- ↑ B. Böttcher, L. Wildt: Gynäkologische Endokrinologie. Diagnosis and therapy . tape 14 , no. 3 , September 2016, p. 212-216 , doi : 10.1007 / s10304-016-0088-9 .