Cortisol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
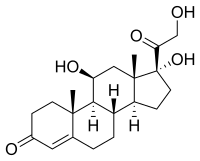
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Hydrocortisone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 30 O 5 | ||||||||||||||||||
| Brief description |
bitter-tasting, colorless flakes |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 362.47 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
212-213 ° C |
||||||||||||||||||
| solubility |
Slightly soluble in water, easily soluble in dioxane |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cortisol or cortisol (also hydrocortisone and hydrocortisone ) is a stress hormone that activates catabolic (= degrading) metabolic processes and thus provides the body with high-energy compounds. Its dampening effect on the immune system is often used in medicine to suppress excessive reactions and to inhibit inflammation .
Cortisol belongs to the group of glucocorticoids . The formation of cortisol in the zona fasciculata of the adrenal cortex is stimulated by the so-called adrenocorticotrope hormone (ACTH) from the anterior pituitary gland (ad-reno-cortico-trop = directed at the adrenal cortex). Overactive leads to the clinical picture of Cushing's disease , while underactive is called Addison's disease . In addition, cortisol is involved in the regulation of growth.
discovery
In the 1930s, American and European researchers looked independently at drugs secreted by the adrenal glands. The substances sought for because of their hormonal effects occur in tiny amounts in the organs. Direct structural elucidation was impossible with the analytical methods available at the time. Edward Calvin Kendall and co-workers therefore had to extract glands from 1.25 million cattle from slaughterhouses. From the extract they isolated u. a. eight steroids, which were initially called Compounds AH . Kendall then named his Compound E cortisone; Compound F was later referred to as cortisol. Tadeus Reichstein was able to explain the chemical structure and configuration of the substance. The chiral substance can be formally derived from the steroid hydrocarbon pregnane , which results in the numbering of the carbon atoms. It contains three hydroxyl groups and two keto groups , one of which is α, β-unsaturated (conjugated), an enone structure. The hydroxy groups are classified as primary, secondary, and tertiary, which is reflected in the reactivity.
 Pregnan with numbering of the carbon atoms. The CH 3 groups and the CH 3 CH 2 group are β-configured; H. point to the viewer.
Pregnan with numbering of the carbon atoms. The CH 3 groups and the CH 3 CH 2 group are β-configured; H. point to the viewer.
biosynthesis
Cortisol is a steroid hormone found in the adrenal cortex. It arises from cholesterol and is therefore derived from isopentenyl pyrophosphate (IPP). The first synthesis of pregnenolone takes place in the mitochondria of the adrenal cortex , a common precursor of steroid hormones (e.g. cortisol), mineralocorticoids (e.g. aldosterone), androgens (e.g. testosterone ) and estrogens (e.g. , cortisol) . Estradiol). The enzyme that catalyzes the formation of pregnenolone via interconnect 20 α , 20 β catalyzes -Dihydroxycholesterin called Cholesterindesmolase and is a monooxygenase , the NADPH as a cofactor needed. During this six-electron oxidation , three NADPH and three oxygen molecules are consumed. It needs the heme-containing cytochrome P450 as a coenzyme . However, the rate-limiting step in cortisol biosynthesis is not cholesterol desmolase, but a cholesterol translocase, which is located on the outer mitochondrial membrane and transports the cholesterol required on the inner mitochondrial membrane. This protein, known as “ StAR protein ” (steroidogenic acute regulatory protein), is increasingly expressed in the presence of cAMP (cyclic adenosine monophosphate ) and is therefore the regulatory step in which ACTH influences cortisol synthesis, among other things.

Pregnenolone leaves the mitochondrion and is converted into progesterone by a 3 β -hydroxysteroid dehydrogenase and an isomerase . In the endoplasmic reticulum (ER), this progesterone is converted into 17 α -hydroxyprogesterone by the enzyme 17-steroid hydroxylase . By a further hydroxylation under catalysis of 21-hydroxylase which then again in the mitochondrion by the formed 11-desoxycortisol, 11 steroid β -hydroxylase is converted to cortisol. All the enzymes described are specific iron-containing cytochrome P450 enzymes . If the enzymes involved in cortisol biosynthesis (mostly 21-hydroxylase) are defective, the secretion of ACTH is not inhibited by negative feedback through cortisol, and the precursors of 11-deoxycortisol accumulate. These can now be used increasingly for the synthesis of androgens, and the clinical picture is an adrenogenital syndrome .
Chemical syntheses
For further pharmacological studies it was imperative to develop chemical syntheses. Although these were pioneering achievements for the "art of organic synthesis", they were very complex and resulted in very low yields. However, previous studies of biosynthesis had shown that corticoids are formed in vivo from progesterone by hydroxylating both C-11 and the side chain. Progesterone can be obtained from steroids of plant raw materials, e.g. B; Sitosterol. This led researchers from the US company Upjohn to search for organisms in the ground that made such hydroxylations possible through their metabolism. Rhizopus arrhizus and R. nigricans were able to convert progesterone into 11α-hydroxyprogesterone.
A synthesis was based on this reaction, which reached the first target molecule (acetylcortisol) in seven further steps. The substance is usually referred to as cortisol acetate or hydrocortisone acetate - not in accordance with the nomenclature .
Although the side chain at C-17 in progesterone also consists of two carbon atoms, as in the target molecule, the hydroxylation of C-17 and C-21 was not easy to achieve. In addition, the α-configuration of the hydroxyl group at C-11 had to be inverted to beta (β). The strategic intention was to brominate the methyl group of the ketone (C-21) and to achieve the α, β-unsaturated carboxylic acid or its ester through a Faworski rearrangement . In order to selectively brominate the methyl group, it was activated by generating an enolate using the Claisen condensation principle with diethyl oxalate. This could be converted into the desired dibromoketone by reaction with two equivalents of bromine , which, by treatment with sodium methoxide in the same reaction vessel, yielded the steroid ester.
This should now be reduced with lithium aluminum hydride to alcohol, which has a further usable allyl structure. For the reduction it was necessary to protect the keto group at C-3. The dioxolane protective group was used for this. During the conversion, however, a deprotonation took place at C-6 ( tautomerism of the conjugated enone system), which was, however, irrelevant for the following reaction steps.
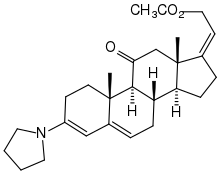
Instead of protection via dioxolane, the reaction of the ketone with pyrrolidine was also investigated, in which a conjugated dienamine is formed with trigonal centers at C-3, C-4, C-5 and C-6. During its hydrolysis, the enone system is also reformed.
The HO group at C-21 was protected with acetic anhydride . The CC double bond between C-17 and C-20 could be oxidized: osmium tetroxide , combined with hydrogen peroxide or phenyliodosoacetate, yielded acetylcortisol (“hydrocortisone acetate”). The latter - itself a pharmaceutical active ingredient - can be "saponified" (hydrolyzed) to cortisol.
Physiological effect
Cortisol has a very broad spectrum of activity and has metabolic effects on the carbohydrate balance (promoting glucose production in the liver and thus counteracting the effects of insulin ), lipid metabolism (promoting the lipolytic effect of adrenaline and noradrenaline ) and protein turnover ( catabolic ). Cortisol has a similar effect to aldosterone and is therefore oxidized to cortisone in the kidneys, intestines and some other tissues , which does not bind to the mineral corticoid receptor and therefore has no antidiuretic effect, i.e. that is, it does not hinder the excretion of toxic substances in the urine . If there is a lack of functional adrenal cortical tissue ( Addison's disease ), cortisol must be substituted.
Cortisol is the most important corticosteroid hormone and vital for humans and higher animals . It is adjacent to the catecholamines an important stress hormone and influences among other blood pressure. However, the cortisol system reacts more slowly than the catecholamine system because, unlike catecholamines and glucagon, it does not work via G-protein-coupled receptors, but via a regulation of gene expression. Here, cortisol binds to the nuclear glucocorticoid receptor . This is activated in its capacity as a transcription factor and leads to the expression of various target genes, e.g. B. from enzymes of gluconeogenesis or from β 2 - adrenoceptors . This explains the effects of cortisol on metabolism. In addition, the cortisol-bound glucocorticoid receptor can also interact directly with other transcription factors (e.g. NF-κB ); this mechanism plays a role in the effect on the immune system (see below).
The higher levels of cortisol release are from the hypothalamus via the pituitary gland . The hypothalamus releases the CRH (corticotropin-releasing hormone), which leads to the release of the adrenocorticotropic hormone ( ACTH ) in the adenohypophysis (anterior pituitary gland) . What is remarkable about these hormones is a pulsatile release, which means that they are released in regular bursts (7-10 per day). The normal morning cortisol levels in the blood serum are 165–690 nmol / l (total cortisol) or 5–23 nmol / l (free cortisol) and show typical fluctuations over the course of the day ( circadian rhythm ). The highest value is reached shortly after waking up in the morning (Cortisol Awakening Response, CAR). Because of the strong circadian fluctuations, a single measurement of cortisol does not make sense. To check the function of the adrenal cortex, it is therefore necessary to determine a daily cortisol profile.
Pharmacological application
In higher doses, cortisol has anti-inflammatory and immunosuppressive effects . Hydrocortisone, as the synthetic form of cortisol is called in pharmacology, is taken orally or injected intravenously for immunosuppression. It must be noted, however, that the effect of the intravenously administered doses clearly exceeds that of the orally administered, since cortisol is metabolized in the liver (glucoronidation and then excretion via the kidneys, see first-pass effect ). However, this method is more effective for conditions with scattered symptoms such as hives .
Hydrocortisone is applied as an ointment to the affected areas of the skin for an anti-inflammatory effect (for example with eczema ) . In the case of joint inflammation (for example due to gout ), the active ingredient can also be injected into the inflamed joint.
If the adrenal cortex is underactive ( Addison's disease ), cortisol is administered as a substitution therapy .
Trade names
- Monopreparations
- Alfacorton (CH), Colifoam (A), Ebenol (D), Eczema Ointment (A), Hydrocortone (A), Hydrocutan (D), Hydroderm (A), Hydrogel (D), Linola Akut (D), Linolacort Hydro (D ), Locoid (CH), Muni (D), Sanadermil (CH), Sanatison (D), Solu-Cortef (CH), Soventol Hydrocort (D), Soventol HydroSpray (D), Systral Hydrocort (D), numerous generics ( D, CH)
- Combination preparations
- Baycuten HC (D), Ciproxin HC (CH), Cortifluid (CH), Daktacort (CH), Dermacalm (CH), Fucidin H (CH), Fucicort (D), Haemocortin (CH), Hydoftal (A), Hydrodexan ( D), Neo-Hydro (CH), Otosporin (A, CH), Pigmanorm (D), Septomixine (CH)
Web links
- Neurolab: cortisol
Individual evidence
- ↑ a b c Entry on hydrocortisone. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ↑ a b Hydrocortisone data sheet from Sigma-Aldrich , accessed on March 23, 2011 ( PDF ).
- ^ Entry on cortisol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ EC Kendall. In: Cold Spring Harbor Quart. Biol. , Vol. 5, 299 (1937)
- ↑ Helvetica Chimica Acta , 20, 953 (1937).
- ↑ Helvetica Chimica Acta , 25, 988 (1942).
- ↑ Helvetica Chimica Acta , 30, 205 (1947).
- ↑ O. Mancera, HJ Ringold, C. Djerassi, G. Rosenkranz, F. Sondheimer. In: Journal of the American Chemical Society , vol. 75, 1286 (1953).