Pregnenolone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Pregnenolone | |||||||||||||||||||||
| other names |
3 β- hydroxypregn-5-en-20-one |
|||||||||||||||||||||
| Molecular formula | C 21 H 32 O 2 | |||||||||||||||||||||
| Brief description |
colorless needles |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 316.48 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
193 ° C |
|||||||||||||||||||||
| solubility |
almost insoluble in water (7.06 mg l −1 at 37 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Pregnenolone is a derivative of the pregnane (C 21 - Steroid ) and is selected from cholesterol formed.
biosynthesis
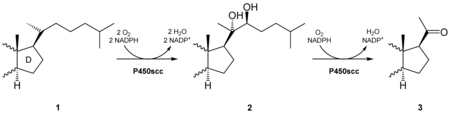
Pregnenolone is formed from cholesterol by hydroxylation at C 20 and C 22 with subsequent cleavage of the side chain . The conversion is catalyzed by the enzyme cholesterol monooxygenase in the mitochondria and controlled by the pituitary hormones ACTH , FSH , LH .
The necessary transport of cholesterol from the cytosol into the mitochondria takes place by binding to the StAR protein , a transport protein in the mitochondrial membrane. Nothing is yet known about the exact transport of pregnenolone from the mitochondria.
Prohormone
Pregnenolone is a prohormone of the steroid hormones and can be further converted to progesterone or 17-hydroxypregnenolone .
Use and therapeutic effectiveness
In animal experiments on mice and rats, pregnenolone blocks the activation of the cannabinoid receptor type 1 (CB1). a. is activated by ∆ 9 - tetrahydrocannabinol (THC), the main active ingredient of hemp ( Cannabis sativa ). It could thus act as an antagonist .
Web links
Individual evidence
- ↑ a b Entry on pregnenolone. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ Entry on pregnenolone in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Data sheet 5-Pregnen-3β-ol-20-one from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ↑ Pregnenolone biosynthesis on Reactome.Org (R-HSA-196108), accessed July 28, 2020
- ↑ Antidote to cannabis high discovered. Der Standard, January 3, 2014, accessed January 5, 2014 .
- ↑ M. Vallee, S. Vitiello et al. a .: Pregnenolone Can Protect the Brain from Cannabis Intoxication. In: Science. 343, 2014, pp. 94–98, doi : 10.1126 / science.1243985 .