Tetrahydrocannabinol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
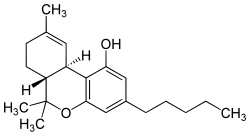
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetrahydrocannabinol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 30 O 2 | ||||||||||||||||||
| Brief description |
colorless oil |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 314.47 g mol −1 | ||||||||||||||||||
| Physical state |
liquid to solid |
||||||||||||||||||
| boiling point |
155-157 ° C (6.65 Pa ) |
||||||||||||||||||
| pK s value |
10.6 |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetrahydrocannabinol [THC, more precisely (-) - Δ 9 - trans -Tetrahydrocannabinol ] is a psychoactive substance that is one of the cannabinoids .
The substance occurs in plants of the genus hemp ( cannabis ) and it is attributed the main part of the intoxicating effect. In the plant, THC is largely in the natural form of two THC acids. These are only converted to THC through decarboxylation , which is achieved by heating the plant material.
The best known natural source of cannabinoids is the resin of the cannabis plant. Marijuana seized by police in Europe contained between 3 and 22% THC in 2015, an average of around 10%.
The American chemist Roger Adams isolated and identified cannabidiol from plant material and thus showed the connection to cannabidiol and tetrahydrocannabinol. In its pure form, THC was first isolated in 1964 by Yehiel Gaoni and Raphael Mechoulam at the Weizmann Institute for Science in Israel. Tetrahydrocannabinol is subject to the provisions of the Narcotics Act in Germany .
chemistry
THC is mainly obtained from the hemp plant (cannabis). The unfertilized female flowers are particularly rich in THC (around 2 to 30%); the THC content of the other parts of the plant is much lower (just under 1%). There is no THC in the seeds of the plant. The leaves near the flower contain around 5 to 6% THC. In contrast to female plants, male plants contain very little THC.
There are four stereoisomers of Δ 9 -tetrahydrocannabinol :
- (-) - Δ 9 - trans -Tetrahydrocannabinol and (+) - Δ 9 - trans -Tetrahydrocannabinol as well
- (-) - Δ 9 - cis -tetrahydrocannabinol and (+) - Δ 9 - cis -tetrahydrocannabinol.
The main psychoactive isomer is the (-) - Δ 9 - trans -THC ( dronabinol ), which is 6 to 100 times more effective than the (+) - Δ 9 - trans -THC. The cis forms have no psychoactive effectiveness; there are different reports about their natural occurrence.
biosynthesis
Tetrahydrocannabinol is in the cannabis plant predominantly as THC acid (THCA, 2-COOH-THC, THC-COOH) it: Through enzymatic condensation of the two precursors geranyl pyrophosphate and Olivetolsäure is cannabigerol formed acid which then enzymatically is rearranged in Tetrahydrocannabinolsäure. The acid partially decarboxylates to THC through heat and UV radiation . A conversion of orally ingested THC-carboxylic acid into THC could not be detected in feeding experiments with rats.
extraction
THC is highly lipophilic . It can be isolated by extraction from THC-containing plant material, for which non-polar and weakly polar solvents such as n - alkanes , acetone , isopropyl alcohol or ethanol are suitable. After the solvent has evaporated, a resinous, oily extract remains . The composition of the extract depends on the choice of solvent. Under suitable conditions, very high THC concentrations of up to 90% can be achieved. This extract is also known as hash oil .
With n -butane , lipophilic ingredients can be extracted from the plant material at very low temperatures; however, this method involves a high risk of fire and explosion. Butane already evaporates at room temperature. The extract obtained in this way has an appearance similar to amber ; at room temperature it is viscous and strings like synthetic resin. If you cool it down, it freezes relatively quickly.
In addition to THC, the extract contains other cannabinoids; When using more polar extraction agents such as ethanol, correspondingly polar substances can be contained, such as chlorophyll , alkaloids ( trigonelline , hordenine ), amino acids , amino sugars , and possibly also undissolved fine parts of the starting material. The extract can be further purified by suitable processes.
Synthesis / partial synthesis
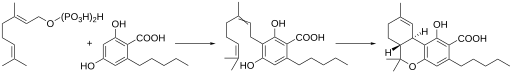
Dronabinol can be synthesized from the terpene limonene, which occurs in citrus fruits , or it can be produced partially synthetically using complex processes from low-THC hemp (extraction of cannabidiol and conversion into THC). It is then much more expensive than if it were extracted from potent medicinal cannabis. The direct extraction of dronabinol from THC-rich varieties (which can have a biosynthetic capacity of 18 to 22%, based on the dry matter of the plant parts) is not possible in many countries for legal reasons. The literature describes the preparation starting from 3,5-dihydroxybenzoic acid as a further possibility of total synthesis . After the functionalization, racemic dronabinol is formed in a heter-Diels-Alder reaction with geranial as the key step.
Uses of cannabis
If THC is ingested through cannabis consumption, the most common form of consumption is smoking hashish or marijuana straight or mixed with tobacco as a joint . Often, THC-containing material is also smoked with the help of special smoking accessories such as bongs and pipes or vaporized and inhaled with a vaporizer .
In addition, THC is also processed in food and drinks. Since THC is lipophilic , it can be processed in high-fat foods such as milk, cakes and muffins. It is not known that users would inject cannabis / THC. Due to its lipophilicity, THC can not be administered intravenously without an emulsifier . Due to its poor solubility in water , it can be administered in the form of solutions or emulsions with ethanol , dimethyl sulfoxide , polysorbate 80 , Cremophor EL or polyvinylpyrrolidone .
pharmacology
Mechanisms of Action

The mechanism of action of THC is not yet fully understood.
THC acts on at least two types of receptors found in mammals, namely CB 1 and CB 2 . CB 1 receptors are mainly located in central and peripheral nerve cells, where they modulate the release of neurotransmitters . But they are also found in other cells, for example in the pituitary gland , immune cells, gastrointestinal tissue, sympathetic ganglia, heart, lungs, urinary bladder and adrenal glands. CB 2 receptors are mainly found in immune cells and are involved in the release of cytokines .
Endocannabinoids are endogenous substances that act on the CB 1 and CB 2 receptors. They are eicosanoids and are produced by the organism when required. The best known are arachidonylethanolamide ( anandamide ) and 2-arachidonylglycerol (2-AG). The endocannabinoids and the cannabinoid receptors form the so-called endocannabinoid system .
THC binds to the CB 1 receptors and influences the signal transmission at these synapses, with effects on the central and peripheral nervous system, such as feeling of happiness, relaxation and analgesia (pain relief). The activation inhibits via G-proteins to adenylate cyclase , blocked Ca 2+ channels and activates K + channels . The transduction mechanisms are similar to the opioid receptor subtypes μ , δ and κ .
Less is known about the role of CB 2 receptors, but it is believed that they are involved in immune modulation because they are predominantly found in B cells and natural killer cells .
In the animal model, THC has an antagonistic effect on 5-HT 3 receptors , which are involved in nausea. THC also acts on other pharmacological targets, such as capsaicin- sensitive perivascular sensory nerves.
The distribution pattern of the CB 1 receptors in the brain determines many of the pharmacological properties of THC. In the brain stem , where vital functions such as breathing are coordinated, these receptors are very few available to no. However , many of these receptors are found in the hippocampus , where short-term memory is located. CB 1 receptors in the basal ganglia explain the influence of THC on motor skills .
In addition to its own therapeutic effects, the weakly psychoactive cannabidiol (CBD) has a modulating influence on THC. Both THC and CBD have an antioxidant effect and thus develop a neuroprotective effect, for example in the case of glutamate- induced excitotoxicity . THC inhibits the release of glutamate, possibly also the entry of calcium via the ion channels , and could therefore develop a neuroprotective effect.
The Δ contained in cannabis in small quantities 8 -tetrahydrocannabinol (Δ 8 -THC) is psychoactive , but slightly less potent than Δ 9 -THC.
THC and CBD can induce signs of apoptotic and necrotic cell death in tumor cells.
metabolism
In humans, Δ 9 -THC is predominantly oxidized to 11-hydroxy-Δ 9 -THC (11-OH-Δ 9 -THC) . This metabolic product is also psychoactive and is further metabolized to 11-nor-9-carboxy-Δ 9 -THC (11-COOH-THC, THC-COOH, THC-carboxylic acid, not psychoactive). Over 100 different Δ 9 -THC metabolites have been identified in humans and animals , almost all of which are not psychoactive. The metabolism takes place mainly in the liver and by the cytochrome P450 enzymes 2C9 , 2C19 and 3A4 . The metabolites are then stored due to its lipophilic properties in adipose tissue from which they then only very slowly removed. More than 65% of the originally present THC is excreted in the form of metabolites in the stool and around 25% in the urine , a small part is broken down in the body itself. The main metabolites in the urine are THC-COOH esterified with glucuronic acid and free THC-COOH, while 11-OH-THC dominates in the stool.
toxicity
The LD 50 in the mouse is 42 mg / kg body weight intravenously and 482 mg / kg in the case of oral administration; in the rhesus monkey , after intravenous administration of 128 mg / kg body weight, death occurs from respiratory arrest and heart failure.
The LD 50 value is not determined in humans and cannot be reliably extrapolated. Assuming a rough (and low) estimate of the potential oral LD 50 value for people with 150 mg / kg body weight, then a 70 kg person after acute oral consumption of 10.5 g THC would have a probability of 50% die. This amount is contained in around 70 to 130 g of a cannabis product with 8 to 15% THC content. Since THC is only absorbed by the intestines to about 6% and by the lungs to about 20% , it is impossible to supply lethal amounts of THC through the consumption of natural cannabis products, especially since the required amount is about a factor of 1000 above the usual consumption amount. Although deaths that occur directly as a result of the ingestion of Δ 9 -THC are rare, there have been studies in the past by forensic medicine at the University Clinic in Düsseldorf that confirm heart failure caused by the direct ingestion of Δ 9 -THC.
Pharmacokinetics
Psychological effects occur with the following dosages: 30 to 50 μg / kg intravenously, 50 μg / kg with smoke inhalation, 120 μg / kg orally. With smoke inhalation of smaller amounts of THC (5 to 7 mg) the sedative component predominates , with amounts of 15 mg or more vigilance predominates , which can increase to psychotic states.
When inhaling smoke, about 20% of the Δ 9 -THC present in the smoke passes into the blood, and only about 6% orally. THC passes from smoke into the blood very quickly; the development of the plasma concentration is comparable with intravenous intake. When taken orally in the form of sesame oil capsules , the effect is reduced due to the first pass effect , the bioavailability is only about 10 to 20%, the highest THC concentration is reached after about two hours.
THC is mainly bound to proteins in the blood plasma ; a maximum of 10% occurs in the red blood cells . The plasma half-life after intravenous administration develops in four phases, suggesting that there are at least four types of tissue into which THC seeps, each with a different permeability and binding capacity. After a sharp decrease in the first few minutes, the THC concentration only drops slowly. The half-lives of the first three phases are 1 minute, 4 minutes and 1 hour, respectively. The initially short half-life is due to the rapid transfer of THC into certain types of tissue and the rapid metabolism of the substance. After about 6 hours there is a pseudo-equilibrium between the THC levels in the blood plasma and in the tissues. The half-life of the fourth phase (terminal half-life after reaching pseudo-equilibrium) is given differently as 19-36 hours. After 5 days around 80 to 90% of the THC is excreted in the form of metabolites, around two thirds in the stool and one fifth in the urine.
The THC concentration in the brain reaches its maximum after around 30 minutes; the concentration is three to six times higher than in plasma. The THC concentration curves in the brain and in the plasma run parallel, which speaks in favor of unrestricted passage through the blood-brain barrier . Animal experiments have shown that THC, as a lipophilic substance, accumulates strongly in certain types of tissue, for example in body fat, heart, liver and lungs. Animal experiments have also shown that THC passes through the placenta to fetuses. The effects of this are largely unknown.
Synthetic analogues
| Active ingredient | effect | Active ingredient | effect |
|---|---|---|---|
| Δ 6a, 10a -THC | possibly psychoactive | Δ 6a, 10a -hexyl-THC | Synhexyl ( parahexyl ), weaker effect than Δ 9 -THC |
| Δ 6a, 10a -Dimethylheptyl-THC | DMH-THC, partly psychoactive | Δ 6a, 10a -methyloctyl-THC | |
| Dimethylheptylpyran | DMHP, CB 1 agonist, more potent than Δ 9 -THC | BRL 4664 | |
| Nabilone | Antiemetic, psychoactive | Levonantradol | Antiemetic, psychoactive |
| HU-210 | psychoactive, 100 to 800 times as potent as Δ 9 -THC | Dexanabinol (HU-211) | not psychoactive ; Dexanabinol is the enantiomer of HU-210 |
| CP-47,497 | Analgesic , psychoactive | CP-55,940 | psychoactive, around 45 times as potent as Δ 9 -THC |
Effects
Known effects of Δ 9 -THC on humans or effects of cannabis that are attributed to Δ 9 -THC: A study from 2019 saw insufficient evidence for an antidepressant effect.
| Effects with therapeutic potential | Effects of intoxication ("high") | Other effects |
|---|---|---|
|
|
|
With regular, intensive consumption, a tolerance effect (necessary dose increase in order to achieve the usual effect) can develop. Withdrawal symptoms and the associated development of dependency are caused by an underfunction of the mesolimbic system ( subcortical reward system), which takes effect after consumption is stopped and lasts until neuronal equilibrium (weaning) is restored in these areas after a maximum of two to three weeks Has.
An increased THC content in illegal cannabis products and an increased consumption behavior is associated with a higher likelihood of developing a psychotic disorder (e.g. schizophrenia). A causal connection has not yet been found. Therefore, it remains unclear whether cannabis occurs here as the sole factor or only in combination with others as a trigger. A disruption of dopaminergic systems caused by cannabinoids was discussed as a possible neurobiological mechanism .
Pure cannabis smoke contains greater amounts of carcinogenic substances than smoke from tobacco , but studies on the relationship between lung cancer and the consumption of pure cannabis are inconclusive. The World Health Organization cites epidemiological evidence that cannabis use increases the risk of testicular cancer by two and a half times, but not that of lung, head and neck cancer. A possible reason is that various factors could contribute, e.g. B. Potential anti-inflammatory and anti-neoplastic properties of THC and other cannabinoids. In contrast, there is a longitudinal study over 40 years with 50,000 Swedish recruits, in which pure cannabis consumption, taking into account all other factors such as tobacco consumption, found an approximately twice as high risk of lung cancer. If cannabis is smoked together with tobacco, for example as a joint or blunt , the risks of nicotine consumption , such as B. the risk of arteriosclerosis , lung diseases such as lung cancer or nicotine addiction .
There is no evidence that THC itself is mutagenic , carcinogenic or teratogenic ( teratogenic ). Pregnant and breastfeeding women as well as adolescents should refrain from consuming THC because damage to the unborn or breastfed child cannot be ruled out and there are indications that THC could have a lasting effect on the development of the immature brain.
Meta-analyzes from 2013 and 2014, which evaluated the brain studies available up to then using imaging methods , came to the result that cannabis consumption in the prefrontal cortex (frontal side of the frontal lobe of the cerebral cortex) leads to a reduced brain volume and to an impairment of the white matter (nerve connections), and a bilateral reduced volume of the hippocampus. In the latter part of the brain, which plays a key role in all memory functions , there was also a correlation (correspondence) between the decrease in volume and the amount of cannabis consumption to date.
Medical application
On May 4, 2016, the Federal Government of Germany passed a draft law that should enable patients to be supplied with natural cannabis and reimbursement by the health insurance companies, and which was unanimously passed by the Bundestag on January 19, 2017. According to the announcement published on March 9, 2017, needy, chronically seriously ill people can get cannabis on prescription, with the costs being partially covered by the health insurance companies. Doctors should independently decide whether cannabis therapy makes sense, even if there are other treatment options in individual cases. "So the patients do not have to be" exhausted ", as it was initially said, before they are entitled to a cannabis prescription."
Dronabinol is in Germany and other countries as a prescription narcotic for making recipe pharmaceuticals available. Under the trade name Marinol ® , it is approved in the United States for the treatment of anorexia and cachexia in AIDS and as an antiemetic in the context of cancer therapy, but the therapy of too high intraocular pressure ( glaucoma ) is not an approved field of application .
The fully synthetic THC analogue nabilone has similar indications as dronabinol. The THC analogue Levonantradol is only used in Germany for research purposes. THC is also in the clinical trial phase for the treatment of glaucoma and autoimmune diseases such as multiple sclerosis , Crohn's disease or ulcerative colitis . The results of a six-week study at the Hannover Medical School confirmed that THC tics are effective in reducing tics in those affected by Tourette's syndrome .
Cannabis flowers (lat. "Cannabis flos" ) are available in the Netherlands in four varieties with different nominal THC contents for human and veterinary medicine: Bedrocan (THC approx. 22%; CBD <1%), Bedrobinol (THC approx , 5%; CBD <1%), Bediol (THC approx. 6.3%; CBD approx. 6%) and Bedica (THC approx. 14%; CBD <1%; ground flowers). The sales price is € 34.50 excl. VAT. indicated for 5 g flowers (as of July 2017). The cannabis is grown in the Netherlands under state supervision, the trade is subordinate to the Bureau voor Medicinale Cannabis (BMC).
In Austria, Canada and Great Britain an oral spray with the trade name Sativex (active ingredient: Nabiximols , consisting of vegetable THC and cannabidiol ) is available for the treatment of neuropathic pain and spasms in multiple sclerosis as well as for the treatment of pain, nausea and vomiting associated with cancer and AIDS diseases approved. Further areas of application are in clinical trials. In Germany, following an amendment to the Narcotics Act in May 2011, the oral spray has been approved as a prescription BTM for the treatment of spasticity in MS since July 1, 2011.
According to a small, placebo-controlled study from 2007, inhalation of THC had a slightly positive effect on neuropathic pain in the context of a polyneuropathy in AIDS.
Drug detection
The detection time for THC in urine is two to 35 days, depending on consumption - even longer in extreme cases - or around 12 hours in the blood. Evidence in the urine is usually made via the THC metabolites THC-carboxylic acid and 11-hydroxy-THC. In addition to the comparatively complex LC / MS method, there are a number of immunassay tests such as radioimmunassay (RIA), enzyme-multiplied immunoassay technique (EMIT), CEDIA ( cloned enzyme donor immunoassay ) and FPIA for the detection of THC metabolites in urine ( fluorescence polarization immunoassay ). To reduce the number of false positive results with these tests, the US Substance Abuse and Mental Health Services Administration (SAMHSA) recommends a cutoff value of 50 ng / ml. The GC / MS method can be used for the highly specific and highly sensitive quantification of the THC carboxylic acid in the fg range . Highly fluorinated derivatives , such as the THC-COOH-HFBA-PFPOH derivative, are measured using the deuterated derivative as an internal standard according to the principle of isotope dilution analysis with the NCI technique (negative chemical ionization). This methodology avoids the problems of false-positive or false-negative analytical results described above, which are observed again and again in enzyme immunassays (ELISA), and is therefore also used in forensic analysis in arbitration analyzes.
False-negative results can be caused by diluted urine samples, for example when diluting in vivo through increased fluid intake. The creatinine content and the osmolality can provide clues about the dilution of the urine , but there is disagreement about the creatinine value from which a urine sample is considered to be “undiluted”.
False-positive results have been reported in some intensive care patients, as well as in people who have given up cannabis use but do more sport: Since THC is stored in adipose tissue, THC metabolites can be released when fat reserves are broken down.
Particular care should be taken when interpreting hair analyzes. As recent studies show, positive measured values cannot necessarily be associated with active cannabis use.
A new analytical procedure for the detection of THC in the breath was developed by NIST employees. The so-called porous layered open tubular cryoadsorption (PLOT cryo) method is used.
Legal
Germany
In Germany, Δ9-THC is classified in Annex II of the Narcotics Act (BtMG) as a marketable, but not prescription-capable narcotic . The (-) - Δ9- trans -THC (dronabinol) used for medical purposes , in the trade as a prescription substance or as a finished medicinal product Marinol (individual import from the USA or Canada possible in accordance with Section 73 (3 ) AMG ), on the other hand, is marketable and prescribable according to the annex III . Isomers of Δ9-THC such as Δ6a-, Δ6a (10a) -, Δ7-, Δ8-, Δ9 (11) - and Δ10-THC are not marketable ( Appendix I ). In Germany , dronabinol is produced by Bionorica Ethics and THC Pharm , two subsidiaries of Bionorica SE . Finished medicinal products containing dronabinol have not yet been approved in Germany.
The statutory health insurances (e.g. AOK) only cover the costs of the medication in about half of the cases, which in individual cases can cost up to 800 euros per month, even if this form of therapy is often the last resort for various clinical pictures is and could be. In 2018, the approval rate from the statutory health insurance companies was around 60 percent, as Bionorica informed.
THC has an effect on the central nervous system , so in the opinion of the legislator, after illegal consumption, the use of machines and driving should be avoided. The police can detect traces of THC during driver controls with a sweat, saliva, hair or urine test or by examining the blood even a long time after consumption. The detection time depends primarily on the respective consumption pattern (duration, type of intake, frequency , dose ) and can be between one week and two months in the urine. At the moment, the legal situation has not yet been clearly decided, but there is a risk of fines of at least 500 euros, driving bans of up to three months and four points in Flensburg . The police on site can only carry out preliminary preliminary tests, the blood sample is later examined in a laboratory and the amount of THC and its breakdown products determined. From a legal point of view, it is an administrative offense as soon as THC is detectable in the blood.
In the decision of the Bavarian VGH of January 25, 2006, Az. 11 CS 05.1711, it says: “The current medical and scientific state of knowledge does not justify an increase in the THC concentration of 1.0 ng / ml in the blood of a motor vehicle driver Road safety risks are to be regarded as secured in the sense of Section 11 (7) FeV ( Driving License Ordinance ) that the person concerned must be forced to withdraw their driving license without further clarification of the facts. In the case of occasional consumption of cannabis and driving with a THC concentration between 1.0 and 2.0 ng / ml, a medical-psychological report must be obtained prior to a possible withdrawal of the driving license in accordance with § 14 Paragraph 1, S. 4 FeV. "( FeV § 11 Abs. 7, FeV § 14 Abs. 1, S. 4, StVG § 3 Abs. 1) This only applies if no driving errors were made. In many cases, the administrative authority (driver's license) orders a test of suitability for driving ( MPU ) as proof of suitability for driving .
Switzerland
In Switzerland , the doctor must apply for a patient-specific special permit from the Federal Office of Public Health (FOPH) for therapy with dronabinol . Since dronabinol is not a compulsory health insurance service, the assumption of costs must be clarified in advance and on a case-by-case basis; with some health insurances you need additional insurance. About 500 patients with anxiety disorders , epilepsy, or Crohn's disease benefited from a medical prescription for cannabidiol; Multiple sclerosis sufferers use the prescription drug Sativex, which contains CBD and THC, to relieve cramps.
In Switzerland, a rapid drug test (“drug wipe”) has been expected in traffic controls since the beginning of 2005 .
Since 2011, cannabis cultivation with a THC content of up to 1% has been permitted in Switzerland, mainly because of the natural fluctuations in hemp plants; previously the limit was 0.3%, but it could not be adhered to on a regular basis. Since then, industrial hemp cultivation has increased in Switzerland.
proof
The method of THC detection in road traffic is controversial because the consumer does not have to be under the direct influence of the drug , but for a positive test it is sufficient to have consumed THC days and weeks beforehand. This applies to all urine tests, as these do not directly detect THC, but rather a breakdown product of THC, tetrahydrocannabinolic acid (THC-COOH, also known as THC-carboxylic acid). Cannabinolic acid no longer has an intoxicating effect. However, it is excreted from the body relatively slowly and at different speeds depending on your constitution and can therefore be detected in the urine for a long time, sometimes even for weeks. The currently most reliable detection method is gas chromatography with mass spectrometry coupling (GC / MS) of derivatives (often as trimethylsilyl derivatives ) of THC carboxylic acid. In contrast, saliva and sweat tests such as the drug tests cited above directly detect THC with sufficient sensitivity.
THC levels
Cannabis flowers
Conventionally grown cannabis flowers contain between 4% and 6% THC on average. If you avoid insemination ( sinsemilla ), the THC content increases to 9% to 12%. On the other hand , marijuana grown under artificial light and specially bred for a high THC content can have an active ingredient content of 10% to 20%. Such varieties began in the USA in the 1970s and they have been continued in the Netherlands in particular since the 1980s. Accordingly, the average THC content of the so-called "Nederwiet" in 2007 was around 20%. Nevertheless, reports of allegedly high THC levels are to be viewed as grossly exaggerated. American researchers pointed out that the extremely low values given for comparison for cannabis confiscated in the 1960s or 1970s (partly below 1%) could be attributed to the fact that at that time the whole plants including stems and leaves were analyzed, whereas today only the actually consumed female inflorescences are examined.
hashish
Hashish contains on average between 10 and 15% THC, although - as with marijuana - the range can be very large: high-quality hashish can also contain over 20% THC. A study by the University of Leiden in 2006 examined eleven marijuana samples from Dutch coffee shops ; the THC content was between 11.7 and 19.1%. Two comparative samples of marijuana from Dutch pharmacies contained 12.2 and 16.5% THC, respectively. Hash oil , which is rarely available on the black market, can have THC levels of 20 to 90% depending on the production method.
Food
The Federal Institute for Risk Assessment (BfR) has assessed the risk of psychogenic and pharmacological effects from the consumption of hemp-containing foods with the usual tetrahydrocannabinol levels determined by the monitoring authorities for all population groups, including children. On the basis of the available data, the BfR comes to the following conclusion: Consumption of hemp-containing foods with the underlying total ∆9-tetrahydrocannabinol (THC) levels can result in the acute reference dose (ARfD) proposed by the European Food Safety Authority (EFSA ) being exceeded ) of 0.001 milligrams (mg) per kilogram of body weight.
literature
- World Health Organization , Expert Committee on Drug Dependence (Ed.): Critical Review of Delta-9-tetrahydrocannabinol (PDF) & Isomers of THC (PDF) . Department of Essential Medicines and Health Products, 2018. (English Abstract: WHO endorses decisions of Expert Committee on cannabis and other substances ).
- Franjo Grotenhermen , Britta Reckendrees: Treatment with cannabis and THC . 2., completely revised. and exp. Edition. Nachtschatten Verlag, 2012, ISBN 978-3-03788-147-7 .
- Franjo Grotenhermen, Kirsten Müller-Vahl : The therapeutic potential of cannabis and cannabinoids. In Deutsches Ärzteblatt International. Volume 109, No. 29–30, 2012, pp. 495–501, doi: 10.3238 / arztebl.2012.0495 .
- Franjo Grotenhermen (ed.): Cannabis and cannabinoids. Pharmacology, toxicology and therapeutic potential. Huber, Bern 2004, ISBN 3-456-84105-1 .
- Franjo Grotenhermen: Hemp as medicine. A practical guide to the use of cannabis and dronabinol. AT-Verlag, Baden 2004, ISBN 3-85502-944-X .
- Roger Pertwee (Ed.): Cannabinoids (= Handbook of Experimental Pharmacology. Volume 168). Springer, Berlin 2005, ISBN 3-540-22565-X .
- Arno Hazekamp: cannabis; extracting the medicine. Leiden University, 2007, ISBN 978-90-90-21997-4 .
Web links
- Self-help network cannabis medicine
- Susanne Uhlenbrock, Claudia Langebrake: From the hippie drug to the drug. In: Pharmazeutische-Zeitung. 21, 2002.
Individual evidence
- ↑ a b c d e f Entry on Tetrahydrocannabinole. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2013.
- ↑ a b Entry on tetrahydrocannabinol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Edward R. Garrett, C. Anthony Hunt: Physicochemical Properties, Solubility, and Protein Binding of Δ 9 -Tetrahydrocannabinol . In: Journal of Pharmaceutical Sciences . tape 63 , no. 7 , 1974, p. 1056-1064 , doi : 10.1002 / jps.2600630705 .
- ↑ InfoCard for (6aR, 10aR) -6,6,9-trimethyl-3-pentyl-6a, 7,8,10a-tetrahydrobenzo [c] chromen-1-ol from the European Chemicals Agency (ECHA), accessed on April 22 2020.
- ^ Robert B. Forney, Glenn F. Kiplinger, Toxicology and Pharmacology of Marijuana . In: Annals of the New York Academy of Sciences . tape 191 , no. 1 , 1971, p. 74-82 , doi : 10.1111 / j.1749-6632.1971.tb13989.x .
- ↑ European Drugs Report 2017 (PDF) Retrieved November 14, 2017 .
- ^ Roger Adams, Madison Hunt, JH Clark: Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp . In: Journal of the American Chemical Society . tape 62 , no. 1 , 1940, p. 196-200 , doi : 10.1021 / ja01858a058 .
- ↑ Yehiel Gaoni, Raphael Mechoulam: Isolation, structure and partial synthesis of an active constituent of hashish . In: Journal of the American Chemical Society . tape 86 , no. 8 , 1964, pp. 1646–1647 , doi : 10.1021 / ja01062a046 .
- ↑ Assessment of dronabinol and its stereo-isomers , 34th meeting of the WHO Expert Committee on Drug Dependence (2006).
- ↑ Lumír Ondřej Hanuš u. a .: Phytocannabinoids: a unified critical inventory. In: Nat. Prod. Rep. 33, 2016, pp. 1357-1392 doi: 10.1039 / C6NP00074F
- ^ J. Jung, MR Meyer, H. H. Maurer, C. Neusüß, W. Weinmann, V. Auwärter: Studies on the metabolism of the Delta9-tetrahydrocannabinol precursor Delta9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in rat using LC- MS / MS, LC-QTOF MS and GC-MS techniques . In: Journal of Mass Spectrometry . tape 44 , no. 10 , 2009, p. 1423-1433 , doi : 10.1002 / jms.1624 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules. Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 420.
- ↑ Laura J. Rovetto, Niccolo V. Aieta: Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. In: The Journal of Supercritical Fluids. 129, 2017, p. 16, doi : 10.1016 / j.supflu.2017.03.014 .
- ↑ a b D. Gloss: An Overview of Products and Bias in Research. In: Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. Volume 12, number 4, October 2015, pp. 731-734, doi : 10.1007 / s13311-015-0370-x , PMID 26202343 , PMC 4604179 (free full text) (review).
- ^ Luigi L. Romano, Arno Hazekamp: Cannabis Oil: chemical evaluation of an upcoming cannabis-based medicine . In: Cannabinoids 2013; 1 (1): 1-11 .
- ↑ WHO Expert Committee on Drug Dependence: Critical Review - Cannabis and cannabis resin. (PDF) World Health Organization, 2018, accessed April 2, 2019 .
- ↑ Eberhard Breitmeier: Alkaloids. Teubner, Stuttgart 1997, ISBN 3-519-03542-1 , pp. 87 ff.
- ↑ John ApSimon (Ed.): The Total Synthesis of Natural Products. Vol. 4, John Wiley & Sons, New York 2009, ISBN 978-0-470-12953-1 , p. 233.
- ↑ Patent DE102005028937 A1, method for the production of dronabinol , accessed March 21, 2017.
- ↑ F. Stehle et al. a .: Heterologous biosynthesis of tetrahydrocannabinolic acid. In: Pharmakon . Volume 5, No. 2, 2017, p. 142.
- ↑ Trachsel, Daniel .: Psychedelic chemistry: Aspects of psychoactive molecules . 4th, compl. newly revised Edition Nachtschatten Verlag, Solothurn 2011, ISBN 978-3-907080-53-5 .
- ↑ a b c Lester Grinspoon, James B. Bakalar: Marijuana, the Forbidden Medicine. Zweiausendeins, Frankfurt am Main 1994, ISBN 3-86150-060-4 .
- ↑ MA Huestis: Pharmacokinetics and metabolism of the plant cannabinoids, Δ⁹- tetrahydrocannabinol, cannabidiol and cannabinol . In: Handbook of Experimental Pharmacology . No. 168 , 2005, p. 657-690 , doi : 10.1007 / 3-540-26573-2_23 .
- ↑ Eberhard Teuscher, Ulrike Lindequist: Biogenic poisons . Akademie-Verlag, Berlin 1988, Lethal doses of THC in mice and rhesus monkeys, p. 65 f .
- ↑ Gabriel G. Nahas: Toxicology and pharmacology of cannabis sativa with special reference to Δ 9-THC . In: Bulletin on Narcotics . tape 24 , no. 2 , 1972, p. 11-27 .
- ↑ Benno Hartung, Silke Kauferstein, Stefanie Ritz-Timme, Thomas Daldrup: Sudden unexpected death under acute influence of cannabis . In: Forensic Science International . tape 237 , April 2014, p. e11 – e13 , doi : 10.1016 / j.forsciint.2014.02.001 ( elsevier.com [accessed on September 9, 2019]).
- ^ The central neuropharmacology of psychotropic cannabinoids . In: Pharmacology & Therapeutics . tape 36 , no. 2-3 , 1988, pp. 189-261 , doi : 10.1016 / 0163-7258 (88) 90106-4 .
- ↑ Klaus Aktories, Ulrich Förstermann, Franz B. Hofmann, Klaus Starke: General and special pharmacology and toxicology. 9th edition. Urban & Fischer, Munich 2006, ISBN 3-437-44490-5 .
- ^ A b C. Nora Chiang, Rao S. Rapaka: Pharmacokinetics and Disposition of Cannabinoids. ( Memento from June 3, 2014 in the Internet Archive ) In: Structure-Activity Relationships of the Cannabinoids. (= NIDA Research Monograph. Volume 79). 1987, p. 173.
- ^ JJ Feigenbaum, F. Bergmann, SA Richmond, R. Mechoulam, V. Nadler, Y. Kloog, M. Sokolovsky: Nonpsychotropic cannabinoid acts as a functional N-methyl-D-aspartate receptor blocker. In: Proceedings of the National Academy of Sciences . Volume 86, Number 23, December 1989, pp. 9584-9587. PMID 2556719 . PMC 298542 (free full text).
- ^ Roger G. Pertwee: Pharmacological and therapeutic targets for Δ 9 -tetrahydrocannabinol and cannabidiol . In: Euphytica . tape 140 , no. 1–2 , 2004, pp. 73-82 , doi : 10.1007 / s10681-004-4756-9 .
- ↑ Louisa Degenhardt: "Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis" doi: 10.1016 / S2215-0366 (19) 30401-8 . Lancet Psychiatry journal, October 28, 2019
- ↑ EB Oleson, JF Cheer: A brain on cannabinoids: the role of dopamine release in reward seeking. In: Cold Spring Harbor perspectives in medicine. Volume 2, Number 8, 2012, p., Doi: 10.1101 / cshperspect.a012229 . PMID 22908200 , PMC 3405830 (free full text) (review).
- ↑ M. Di Forti, D. Quattrone et al. a .: The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. In: The lancet. Psychiatry. [Electronic publication before printing] March 2019, doi : 10.1016 / S2215-0366 (19) 30048-3 , PMID 30902669 (free full text).
- ^ JA McLaren, E. Silins, D. Hutchinson, RP Mattick, W. Hall: Assessing evidence for a causal link between cannabis and psychosis: a review of cohort studies. In: The International journal on drug policy. Volume 21, number 1, January 2010, pp. 10-19, doi: 10.1016 / j.drugpo.2009.09.001 . PMID 19783132 (review).
- ↑ S. Minozzi, M. Davoli, AM Bargagli, L. Amato, S. Vecchi, CA Perucci: An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. In: Drug and alcohol review. Volume 29, Number 3, May 2010, pp. 304-317, doi: 10.1111 / j.1465-3362.2009.00132.x . PMID 20565524 .
- ↑ TH Moore, S. Zammit, A. Lingford-Hughes, TR Barnes, PB Jones, M. Burke, G. Lewis: Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. In: Lancet. Volume 370, Number 9584, July 2007, pp. 319-328, doi: 10.1016 / S0140-6736 (07) 61162-3 . PMID 17662880 .
- ↑ R. Kuepper, PD Morrison, J. van Os, RM Murray, G. Kenis, C. Henquet: Does dopamine mediate the psychosis-inducing effects of cannabis? A review and integration of findings across disciplines. In: Schizophrenia research. Volume 121, number 1–3, August 2010, pp. 107–117, doi: 10.1016 / j.schres.2010.05.031 . PMID 20580531 .
- ↑ M. Underner, T. Urban, J. Perriot, I. de Chazeron, JC Meurice: Cannabis smoking and lung cancer. In: Revue des maladies respiratoires. Volume 31, number 6, June 2014, pp. 488-498, doi: 10.1016 / j.rmr.2013.12.002 . PMID 25012035 (Review).
- ↑ M. Joshi, A. Joshi, T. Bartter: Marijuana and lung diseases. In: Current opinion in pulmonary medicine. Volume 20, number 2, March 2014, pp. 173-179, doi: 10.1097 / MCP.0000000000000026 . PMID 24384575 .
- ^ National Institute on Drug Abuse: What are marijuana's effects on lung health? Retrieved April 2, 2019 .
- ↑ WHO Expert Committee on Drug Dependence: Critical Review - Cannabis and cannabis resin. (PDF) World Health Organization, 2018, accessed April 2, 2019 .
- ↑ Luis Ribeiro, Philip W. Ind: Marijuana and the lung: hysteria or cause for concern? In: Breathe . tape 14 , no. 3 , September 2018, p. 196–205 , doi : 10.1183 / 20734735.020418 , PMID 30186517 , PMC 6118880 (free full text).
- ^ Russell C. Callaghan, Peter Allebeck, Anna Sidorchuk: Marijuana use and risk of lung cancer: a 40-year cohort study . In: Cancer Causes & Control . tape 24 , no. 10 , October 2013, p. 1811-1820 , doi : 10.1007 / s10552-013-0259-0 .
- ↑ World Health Organization , Expert Committee on Drug Dependence (Ed.): Critical Review of Cannabis and cannabis resin (PDF) , Department of Essential Medicines and Health Products, Section 3: Toxicology, 1.6 Fertility and teratogenesis, p. 6, 2018.
- ↑ J. Wrege, A. Schmidt, A. Walter, R. Smieskova, K. Bendfeldt, EW Radue, UE Lang, S. Borgwardt: Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. In: Current pharmaceutical design. Volume 20, number 13, 2014, pp. 2126-2137. PMID 23829358 , PMC 4052819 (free full text).
- ↑ A. Batalla, S. Bhattacharyya, M. Yücel, P. Fusar-Poli, JA Crippa, S. Nogué, M. Torrens, J. Pujol, M. Farré, R. Martin-Santos: Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. In: PloS one. Volume 8, number 2, 2013, p. E55821. PMID 23390554 , PMC 3563634 (free full text).
- ↑ Change in the law on narcotics: cannabis for the seriously ill on prescription. Federal Government, May 4, 2016, accessed December 27, 2016 .
- ↑ Götz Hausding: Bundestag approves cannabis medicines for seriously ill patients . In: German Bundestag . ( bundestag.de [accessed on May 12, 2017]).
- ↑ KR Müller-Vahl u. a .: Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. In: J Clin Psychiatry. 2003. PMID 12716250 .
- ↑ Frederike K. Engels, Floris A. de Jong a. a .: Medicinal cannabis in oncology. In: European Journal of Cancer. 43, 2007, pp. 2638-2644, doi: 10.1016 / j.ejca.2007.09.010 .
- ^ Product portfolio of the Dutch Bureau voor Medicinale Cannabis (Dutch).
- ↑ Twenty-fifth ordinance amending the narcotics law ( BGBl. 2011 I p. 821 )
- ↑ Sativex® approved in Germany for the treatment of spasticity due to multiple sclerosis. ( Memento of March 6, 2016 in the Internet Archive ) GW-Pharmaceuticals, May 26, 2011, accessed March 29, 2012.
- ↑ DI Abrams, CA Jay, SB Shade, H. Vizoso et al. a .: Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. In: Neurology. February 13, 2007, Volume 68, No. 7, pp. 515-521.
- ^ Sandra B. Grauwiler, Jürgen Drewe, André Scholer: Sensitivity and Specificity of Urinary Cannabinoid Detection with Two Immunoassays After Controlled Oral Administration of Cannabinoids to Humans . In: Therapeutic Drug Monitoring . tape 30 , no. 4 , 2008, p. 530-535 , doi : 10.1097 / FTD.0b013e318180c7c2 .
- ↑ H.-U. Melchert, H.-J. Hübschmann, E. Pabel: Analysis of THC carboxylic acid. Specific detection and highly sensitive quantification in urine by NCI-GC / MS. In: LABO. Issue 1, 2009, pp. 8–12, ( PDF ).
- ^ Richard A. Gustafson et al. a .: Urinary cannabinoid detection times after controlled oral administration of Δ 9 -tetrahydrocannabinol to humans . In: Clinical Chemistry . tape 49 , no. 7 , 2003, p. 1114-1124 , doi : 10.1373 / 49.7.1114 .
- ↑ Gabriele Halwachs-Baumann: Laboratory Medicine. Clinic, practice, case studies. Springer, Vienna 2006, ISBN 3-211-25291-6 .
- ^ B. Moosmann, N. Roth, V. Auwärter: Finding cannabinoids in hair does not prove cannabis consumption. In: Sci Rep. 5, Oct 7, 2015, p. 14906. PMID 26443501 .
- ↑ TM Lovestead and TJ Bruno, Determination of Cannabinoid Vapor Pressures to Aid in Vapor Phase Detection of Intoxication. Forensic Chemistry. Published online 27 June 2017. PMID 29266138 .
- ↑ Increase in sales: Bionorica can rely on core brands , Pharmazeutische Zeitung, March 2, 2018.
- ↑ Therapy with cannabinoids Therapy with cannabinoids ( Memento from June 3, 2014 in the Internet Archive ), Hänseler AG, accessed October 5, 2011.
- ↑ a b Rebekka Haefeli: Now comes cannabis light. A huge disappointment for stoners, the great hope for planters: low-THC cannabis. The trade in the herb could develop into a billion dollar business. In: Observer. Zurich, July 7, 2017, pp. 36–40.
- ↑ S. Strano-Rossi, F. Molaioni, F. Botrè: Rapid screening of drugs of abuse and their metabolites by gas chromatography / mass spectrometry: application to urinalysis . In: Rapid Communications in Mass Spectrometry . tape 19 , no. 11 , 2005, p. 1529-1535 , doi : 10.1002 / rcm . 1942 .
- ↑ a b c d e Leslie L. Iversen: The Science of Marijuana. Oxford University Press, 2007, ISBN 978-0-19-979598-7 . P. 10.
- ↑ Arno Hazekamp: An Assessment of the Quality of Medical Cannabis in the Netherlands. In: Cannabinoids. Volume 1, No. 1, 2006, pp. 1-10. ( PDF; 278 kB ).
- ↑ Federal Institute for Risk Assessment: Tetrahydrocannabinol levels are too high in many hemp-containing foods - health problems are possible. (PDF) November 8, 2018, accessed November 15, 2018 .