HU-210
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | HU-210 | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 25 H 38 O 3 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 386,567 g · mol -1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
HU-210 is a synthetic cannabinoid . The abbreviation HU stands for Hebrew University , where the compound was synthesized by Raphael Mechoulam's group . HU-210 is 100 to 800 times more effective than the natural tetrahydrocannabinol from the hemp plant and has a longer duration of action. HU-210 is the (-) - 1,1-dimethylheptyl analogue of 11-hydroxy-Δ 8 -tetrahydrocannabinol and is also called 1,1-dimethylheptyl-11-hydroxytetrahydrocannabinol.
chemistry
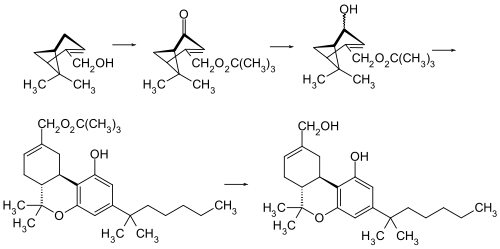
HU-210 is the enantiomer of HU-211 (dexanabinol), which however does not show any cannabinoid effects. The original synthesis of HU-210 is based on an acid-catalyzed condensation of (-) - Myrtenol and 1,1-dimethylheptylresorcinol (3,5-dihydroxy-1- (1,1-dimethylheptyl) benzene).
pharmacology
HU-210 activates both the cannabinoid receptor CB 1 and CB 2 , it has a higher affinity for the CB 1 receptor. In contrast to Δ 9 - tetrahydrocannabinol , the main active ingredient in cannabis, however, HU-210 is a full agonist .
use
HU-210 is used to research the role of the cannabinoid system. According to the United States Customs and Border Protection , HU-210 was found in Spice Gold , which was seized at the US border in 2009. In 2009, HU-210 was detected in three other Spice products in England.
literature
- Razdan, K .: The Total Synthesis of Cannabinoids . In: John Apsimon (Ed.): The Total Synthesis of Natural Products . Wiley Interscience, 1981, ISBN 978-0-471-05460-3 , p. 245.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Little PJ, Compton DR, Mechoulam R, Martin BR: Stereochemical effects of 11-OH-delta 8-THC-dimethylheptyl in mice and dogs . In: Pharmacol Biochem Behav . . 32, No. 3, March 1989, pp. 661-6. PMID 2544901 .
- ^ R. Mechoulam, N. Lander, A. Breuer, J. Zahalka: In Synthesis of the Individual, Pharmacologically Distinct, Enantiomers of a Tetrahydrocannabinol Derivative. Tetrahedron: Asymmetry 1990 , 5 , 315-318.
- ^ Fields CC, Joyce KE, Briley EM, et al. : Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors . In: Mol Pharmacol . 48, No. 3, September 1995, pp. 443-50. PMID 7565624 .
- ^ Ottani A, Giuliani D: Hu 210: a potent tool for investigations of the cannabinoid system . In: CNS Drug Rev . 7, No. 2, 2001, pp. 131-45. PMID 11474421 .
- ^ Lab Results Confirm CBP in Ohio Discover Synthetic Narcotics in Incense Packets . CBP . Archived from the original on February 26, 2013. Retrieved January 2, 2011.
- ↑ EMCDDA Action on new drugs briefing paper: Understanding the 'Spice' phenomenon (PDF; 563 kB) Retrieved January 2, 2011.