Amino sugar
Aminosugars are monosaccharides ( simple sugars ) in which one or more hydroxyl groups (–OH) have been formally replaced by an amino group (–NH 2 ). Free amino sugars are strongly basic and do not occur in nature; the monosaccharides are always components of glycosides and polymeric substances. As building blocks of various, often essential biomolecules , they are fundamental for many central chemical processes and molecular structures in nature.
If the nitrogen is bound to the carbon atom of the anomeric center of a monosaccharide, the amino sugar in question is called glycosylamine , otherwise it is called aminodeoxy sugar .
This group of substances has been discovered in 1875 by the young medical Georg Ledderhose when he scissors and tanks of a recently consumed lobster einkochte in hydrochloric acid and then glittering crystals ( D -glucosamine - hydrochloride discovered). The first description followed the following year, the first synthesis in 1903 (by Fischer and Leuchs ), and in 1914 Phoebus Levene and FB La Forge discovered D- galactosamine . The detection of amino sugars in the influenza virus took place in 1947 (by CA Knight).
Function and meaning
In the nature
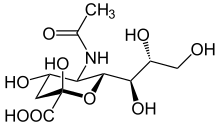
Amino sugars are important in a wide variety of organisms as part of their cell structure : for example, they strengthen the cell wall of bacteria with murein , act as a building block of chitin in arthropods and fungi and represent the group of glycosaminoglycans , which are fiber materials and components of cell membranes in many higher living organisms and the basic substance of the extracellular matrix , which are also present in blood and human milk . In general, 2-deoxy-2-amino aldoses such as D- glucosamine or D- galactosamine are the most important amino sugars. However, even with nucleotides , the coding basic building blocks of DNA , it is a question of substituted glycosylamines.
Due to their amino groups, amino sugars are also a relevant factor for the nitrogen content of soils : an estimated 5–10% of the soil nitrogen is due to them, the majority of which comes from dead microorganisms , whereas plants do not produce amino sugar in significant quantities. In intensively cultivated soils, the proportion of aminosugar is measurably reduced, as bacterial synthesis decreases significantly as a result of industrial land use; In addition, differences in the proportion of certain amino sugars occur between differently cultivated soils (between those of bacterial origin, the concentration of which falls more sharply as a result of arable use, and those derived from chitin), which means that the relationship between the two groups can be used as an indicator for land use influences.
use

A number of glycosidically bound amino sugars are characteristic of aminoglycosides , which act as antibiotics on the protein synthesis of bacteria in such a way that proteins of nonsensical composition are then formed. In addition, sialic acid analogs are used as neuramidase inhibitors to combat viral diseases such as influenza , but these are not themselves sugars . Furthermore, the physiologically in the will mast cells formed cofactor of the anticoagulant , antithrombin III , heparin as anticoagulant used.
With the Amadori rearrangement can be obtained from aldose - N - glucosides corresponding ketose - N glucosides gain, which are important intermediates in the manufacture of osazones , Osonen , quinoxalines and vitamins Riboflavin (B 2 ) and folic acid (B 9 are).
Since amino sugars show a particularly strong magnetic coupling , it has also been proposed to use them - instead of the previously used proteins - as coordination partners for the metal centers of synthetic catalysts .
chemistry
Free amino sugars have a strongly basic reaction, which is why they are acetylated in living organisms . Naturally, amino sugars from hexoses , which include mannose , glucose (grape sugar) and galactose, are particularly common . Since the hydroxyl group of the C2 atom is preferably replaced, 2-amino-hexoses are often formed: glucosamine is formed from glucose, mannosamine from mannose and galactosamine from galactose.
biosynthesis
The biosynthesis of the amino sugars takes place via a transamination , whereby the amino group comes from the amino acid glutamine . For example, glucose-6-phosphate is aminated to D- glucosamine-6-phosphate by the enzyme hexose phosphate transaminase . The glucosamine phosphate formed can be converted into the N-acetyl derivative with the aid of a transacetylase. After conversion to 1-phosphate, this is activated by reaction with UTP and then incorporated into a wide variety of polymer compounds.
Laboratory synthesis
There are several ways to make amino sugars. A method is a ring-closing reaction from the aldol between dialdehydes and nitroalkanes . For example, sucrose can first be split oxidatively with lead (IV) acetate and acetic acid on the ring of the fructofuranose subunit , then closed again via reaction with nitromethane in the presence of sodium methoxide and methanol , and then the nitro group can be reduced to the amino group .
A more recent synthesis route for aminodeoxy sugars is the synthesis of 1-hydroxy-1,2-benziodoxol-3 (1 H ) -one-1-oxide (“IBX”) mediated from glycals .
Analytics
2-acetamido sugars can be detected by the Morgan-Elson reaction . Furans are formed under basic conditions , which are then reacted with Ehrlich's reagent .
literature
- Debenham et al .: Recent Advances in N-Protection for Amino Sugar Synthesis . In: Liebigs Annalen, year 1997, issue 5, pp. 791–802.
- Suzuki et al .: Rapid analysis of amino sugars by microchip electrophoresis with laser-induced fluorescence detection . In: Electrophoresis, Volume 22, Issue 18, pp. 4023-4031.
Individual evidence
- ^ A b Peter, Vollhardt: Organic Chemistry , Wiley-VCH, Weinheim 1990. ISBN 3-527-26912-6 , p. 1102.
- ↑ Kuhn et al .: Amino sugar . In: Angewandte Chemie, year 69 (1957), issues 1–2, pp. 23–33, especially p. 26.
- ↑ a b Ternes, Täufel, Tunger, Zobel: Lexicon of food and food chemistry. Pp. 74-79. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2007. ISBN 978-3-8047-2275-0 .
- ↑ Entry on amino sugar. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Roberts et al .: Free amino sugar reactions in soil in relation to soil carbon and nitrogen cycling . In: Soil Biology and Biochemistry, 39 (2007), pp. 3081-3092.
- ^ Zhang et al .: Amino sugars in soils of the North American cultivated prairie . In: Journal for Plant Nutrition and Soil Science, Volume 160 (1997), Issue 5, pp. 533-538.
- ↑ Bruice: Organic Chemistry . S. 1150. Pearson Studium, Munich 2007. ISBN 978-3-8273-7190-4 .
- ^ Lüllmann, Mohr, Hein: Pocket Atlas of Pharmacology . Pp. 280-281,290,150. Thieme, Stuttgart 2004. ISBN 3-13-707705-2 .
- ↑ Entry on Amadori rearrangement. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Wegner et al .: New building blocks for the design of multicore copper complexes based on amino carbohydrates . In: Angewandte Chemie, Volume 112 (2000), Edition 3, pp. 608–612.
- ↑ Science Online Lexicon: Entry on amino sugar in the Lexicon of Chemistry .
- ↑ Löffler, Petrides, Heinrich: Biochemistry and Pathobiochemistry . Pp. 544-545. Springer, Heidelberg 2007. ISBN 3-540-32680-4 .
- ↑ Shing: Glycol Cleavage Reactions . In: Trost, Fleming (Ed.): Comprehensive organic synthesis: selectivity, strategy and efficiency in modern organic chemistry . P. 712, Volume 7. Pergamon Press, 1991. ISBN 0-08-035929-9 .
- ^ Nicolaou et al .: Novel IBX-Mediated Processes for the Synthesis of Amino Sugars and Libraries Thereof . In: Angewandte Chemie, Volume 112 (2000), Edition 14, pp. 2625-2629.
- ↑ Michael Sinnott: Carbohydrate Chemistry and Biochemistry. Royal Society of Chemistry, 2013, ISBN 978-1-782-62632-9 . Pp. 721, 722.

