glucose
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|

|
|||||||||
|
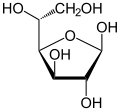
D -glucose (left) and L -glucose (right)
Fischer projection , open-chain representation |
|||||||||
| General | |||||||||
| Surname | glucose | ||||||||
| other names | |||||||||
| Molecular formula | C 6 H 12 O 6 | ||||||||
| Brief description |
D -glucose: |
||||||||
| External identifiers / databases | |||||||||
|
|||||||||
| Drug information | |||||||||
| ATC code | |||||||||
| properties | |||||||||
| Molar mass | 180.16 g mol −1 | ||||||||
| Physical state |
firmly |
||||||||
| density |
1.562 g cm −3 |
||||||||
| Melting point | |||||||||
| solubility |
good in water (470 g l −1 at 20 ° C) |
||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Glucose (abbreviation: Glc ) or glucose (from the Greek γλυκύς 'sweet', and -ose as a suffix for sugar) is a naturally occurring carbohydrate . Of the glucose enantiomers , D -glucose is the natural form. It is also known as grape sugar or, in the case of food ingredients, as dextrose and is the most common monosaccharide (simple sugar). L -glucose can be represented synthetically, but is of little importance (for an explanation of the designations “ D ” and “ L ” see Fischer projection ). If glucose is mentioned without any additional name ( prefix ), D -glucose is meant.
In living organisms, glucose is the main source of energy. It is stored as polymeric glucan , in animals as glycogen and in plants as starch . As a component of cellulose , it is the main component of plant cell walls and thus the most common carbohydrate. Glucose is used in medical infusion solutions and is considered an essential drug .
history
Glucose was first isolated from raisins by Andreas Marggraf in 1747 . It was isolated from grapes by Johann Tobias Lowitz in 1792 and recognized as different from cane sugar ( sucrose ). Glucose is the term coined by Jean Baptiste Dumas in 1838, which has established itself in the chemical literature. By Friedrich August Kekule dextrose proposal comes (from Latin dexter = right), as glucose in aqueous solution the plane of linearly polarized light to the right turns. In contrast, D - fructose (a ketohexose ) and L- glucose rotate linearly polarized light to the left . The earlier d and l nomenclature based on this was abandoned in favor of the D and L notation, which refers to the absolute configuration of the asymmetric center farthest from the carbonyl group, and that with that of D or L -Glyceraldehyde matches.
The structure of glucose and the structural relationships to the other monosaccharides were described by Emil Fischer between 1891 and 1894 using the Fischer projection and represented a milestone in natural product chemistry , for which he received the Nobel Prize in Chemistry in 1902 . His first successful glucose synthesis confirmed the theories of Jacobus Henricus van 't Hoff on the tetrahedral arrangement of chemical bonds in organic carbon compounds and on chemical kinetics . The knowledge obtained in this way enabled the introduction of a systematic nomenclature for the stereoisomeric carbohydrates using the conventional names with reference to their spatial symmetry (e.g. Fischer nomenclature, D / L nomenclature).
Otto Meyerhof received the Nobel Prize for Physiology or Medicine in 1922 for his discovery of the metabolism of glucose . Hans von Euler-Chelpin , together with Arthur Harden, received the Nobel Prize in Chemistry in 1929 for their research on “sugar fermentation and the role of enzymes in this process”. In 1947, Carl and Gerty Cori received the Nobel Prize in Physiology or Medicine for their discovery of the recycling of lactic acid produced in muscle by glycolysis for gluconeogenesis and glycogen synthesis in the liver, and Bernardo Houssay for his discovery of the role of pituitary hormones in glucose metabolism. Luis Leloir received the Nobel Prize in Chemistry in 1970 for his discovery of the sugar nucleotides formed from glucose during the biosynthesis of carbohydrates .
properties
Glucose is usually in solid form as a mono hydrate closed pyran ring before ( Hydratdextrose ). In aqueous solution, on the other hand, it is open-chain to a small extent and is predominantly in the form of α- or β-pyranose, some of which merge through mutarotation. The three known forms can be crystallized from aqueous solutions: α-glucopyranose, β-glucopyranose and β-glucopyranose hydrate. Glucose is a component of the double sugar lactose (milk sugar) and sucrose (cane or beet sugar) and of multiple sugars such as raffinose and multiple sugars such as starch and amylopectin , glycogen or cellulose . The glass transition temperature of glucose is 31 ° C and the Gordon-Taylor constant (an experimentally determined constant for predicting the glass transition temperature at different mass fractions of a mixture of two substances) is 4.5.
Glucose is probably the most common natural monosaccharide because it reacts less with proteins via glycation than other monosaccharides. Another hypothesis is that with glucose in the form of β-D-glucose as the only D-aldohexose all five hydroxy substituents are in the equatorial position and are therefore more easily accessible for chemical reactions, for example for esterification or acetal formation. For this reason, D-glucose is also the most preferred building block in natural polysaccharides (glycans).
Systematics of glucose
| Different forms and representations of D- glucose in comparison | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
 α- D -glucofuranose |
 β- D -glucofuranose |
 α- D -glucopyranose |
 β- D -glucopyranose |
|
| α- D -glucopyranose in (1) Tollens / Fischer (2) Haworth (3) armchair representation (4) stereochemical view | ||

|
||
The illustration of the pyranoid form shown above - in the example α- D -glucopyranose - is called the Haworth projection . The ring is shown as flat, which does not correspond to reality, but is sufficient for many purposes. As a result of the ring closure, the first carbon atom becomes a new, further center of chirality , which forms a pair of anomers . The structure in which the hydroxyl function of the hemiacetal points downwards (axially) in the Haworth projection is referred to as α- D -glucose, that with the hydroxyl function upwards (equatorially) as β- D -glucose. In general, in the α form the hydroxy function formed during ring closure is on the opposite side of the ring plane of the Haworth projection than the hydroxymethylene group (carbon atom 6), in the β form on the same. In this form, the definition applies to D and L sugars as well as to aldoses and ketoses. α- and β- D -glucose are examples of structures called anomers. Anomers are stereoisomeric sugars that differ only in the configuration at the center of chirality formed during ring closure. Anomers are therefore a special case of epimers .
The Fischer projection is confusing for the cyclic hemiacetal forms, see 1. In order to clarify the angled arrangement of the carbon chain, armchair representation 3 is selected. Representation 4 is also common and stereochemically clear.
Behavior in aqueous solution

The ring can be opened and closed in aqueous solution so that there is an equilibrium between pyranose (six-membered ring with endocyclic oxygen atom, 99.75%), furanose form (five-membered ring, in traces) and open-chain aldehyde form (0.25%). The addition of acid or lye accelerates this process. Since either the alpha or beta form can arise during ring closure, there is also a balance between alpha form (36%) and beta form (63.9%). The balance is, as can be seen at the percentages, on the side of β- D -glucose. It is the more stable anomer , since all hydroxyl groups are arranged equatorially in the chair conformation and are therefore as far apart as possible. The fact that the α-anomer is still 36% despite the axial OH group suggests that there must be other influences. The relative stability of the α configuration is called the anomeric effect . Without the anomeric effect, the ratio of α-form to β-form would be 11% to 89%.
Mutarotation
The conversion between the two anomers may be in the polarimeter be observed because pure α- D -glucose a specific angle of rotation of + 112.2 ° · ml · dm -1 · g -1 , has pure β- D -glucose of +17, 5 ° ml dm −1 g −1 . If equilibrium has been established after a certain time, there is an angle of rotation of + 52.7 ° · ml · dm −1 · g −1 . This change in the angle of rotation is known as mutarotation . This conversion can be massively accelerated by adding acid or base . The equilibrium is established via the open-chain aldehyde form.

Isomerization
In dilute sodium hydroxide solution, mannose , glucose and fructose are converted into one another ( Lobry-de-Bruyn-Alberda-van-Ekenstein rearrangement ) so that an equilibrium is formed between these isomers . This reaction takes place via an enediol :
Memory aids for stereochemistry
The following donkey bridge is used to memorize the glucose configuration in the Fischer projection : The positions of the hydroxyl groups to the right and left of the carbon chain can be "symbolized" by onomatopoeia of the sound of a martin 's horn ("Ta-Tü-Ta-Ta") . The arrangement of the hydroxyl groups in galactose , on the other hand, can be remembered as blue light (see illustration).
You can use the word "HochBett" (B for beta) to remember which form of glucose is present. If the C-1 hydroxyl group is at the top ("high") in the Haworth projection, then it is the beta form ("bed").
Occurrence
Glucose occurs in all living things because it is a central component of the metabolism in all living things.
| food | Total carbohydrates including fiber |
Total sugar | Fructose | glucose | Sucrose | Fructose / glucose ratio |
Sucrose in% of total sugar |
|---|---|---|---|---|---|---|---|
| fruit | |||||||
| Apple | 13.8 | 10.4 | 5.9 | 2.4 | 2.1 | 2.0 | 19.9 |
| apricot | 11.1 | 9.2 | 0.9 | 2.4 | 5.9 | 0.7 | 63.5 |
| banana | 22.8 | 12.2 | 4.9 | 5.0 | 2.4 | 1.0 | 20.0 |
| Fig , dried | 63.9 | 47.9 | 22.9 | 24.8 | 0.9 | 0.93 | 0.15 |
| Grapes | 18.1 | 15.5 | 8.1 | 7.2 | 0.2 | 1.1 | 1 |
| Umbilical orange | 12.5 | 8.5 | 2.25 | 2.0 | 4.3 | 1.1 | 50.4 |
| peach | 9.5 | 8.4 | 1.5 | 2.0 | 4.8 | 0.9 | 56.7 |
| pear | 15.5 | 9.8 | 6.2 | 2.8 | 0.8 | 2.1 | 8.0 |
| pineapple | 13.1 | 9.9 | 2.1 | 1.7 | 6.0 | 1.1 | 60.8 |
| plum | 11.4 | 9.9 | 3.1 | 5.1 | 1.6 | 0.66 | 16.2 |
| vegetables | |||||||
| Beetroot | 9.6 | 6.8 | 0.1 | 0.1 | 6.5 | 1.0 | 96.2 |
| carrot | 9.6 | 4.7 | 0.6 | 0.6 | 3.6 | 1.0 | 77 |
| paprika | 6.0 | 4.2 | 2.3 | 1.9 | 0.0 | 1.2 | 0.0 |
| onion | 7.6 | 5.0 | 2.0 | 2.3 | 0.7 | 0.9 | 14.3 |
| sweet potato | 20.1 | 4.2 | 0.7 | 1.0 | 2.5 | 0.9 | 60.3 |
| yam | 27.9 | 0.5 | traces | traces | traces | - | traces |
| Sugar cane | 13-18 | 0.2-1.0 | 0.2-1.0 | 11-16 | 1.0 | high | |
| sugar beet | 17-18 | 0.1-0.5 | 0.1-0.5 | 16-17 | 1.0 | high | |
| Grain | |||||||
| Corn | 19.0 | 6.2 | 1.9 | 3.4 | 0.9 | 0.61 | 15.0 |
Industrial production
Glucose is produced industrially from starch by enzymatic hydrolysis using glucose amylase or by using acids , the enzymatic hydrolysis having largely replaced the acid-catalyzed hydrolysis. This creates glucose syrup (enzymatically with over 90% glucose in the dry matter) with an annual worldwide production volume of 20 million tons (as of 2011). This is where the name "starch sugar" was used in the past. Corn , potatoes , rice , wheat , rye , cassava , sweet potato and sago are used as starch sources . The amylases mostly come from Bacillus licheniformis or Bacillus subtilis (strain MN-385), which are more thermostable than the enzymes used previously. Starting in 1982, pullulanases from Aspergillus niger were used in the production of glucose syrup to convert amylopectin into amylose, thereby increasing the yield of glucose. The reaction is carried out at pH 4.6-5.2 and a temperature of 55-60 ° C. Corn syrup has between 20% and 95% glucose in the dry matter. The Japanese form of the glucose syrup Mizuame is made from sweet potato or rice starch. Maltodextrin contains around 20% glucose.
Conversion to fructose
In the USA , corn is used almost exclusively with the help of amylase and glucose isomerase to produce the food sweetness isoglucose , which is a mixture of glucose and fructose (also high fructose corn syrup HFCS). Fructose has a higher sweetness than glucose with the same physiological calorific value of 374 kilocalories per 100 g. The annual worldwide production of isoglucose is eight million tons (as of 2011).
Industrial use
Above all, glucose is used in the production of fructose and in the production of foods containing glucose . In food it is used as a sweetener , as a humectant , to increase volume and to create a softer mouthfeel . Various sources of glucose such as grape juice (for wine) or malt (for beer) are used to ferment into ethanol in the course of the production of alcoholic beverages . Most soft drinks in the US use HFCS-55 (i.e. 55% fructose) while most other HFCS-sweetened foods in the US use HFCS-42. In neighboring Mexico , on the other hand, cane sugar is used as a sweetener in the soft drink Coca-Cola , which has a higher sweetening power. Glucose syrup is also used in the production of sweets such as candies , toffee and fondant . Typical chemical reactions of glucose in dry cooking techniques are caramelization and, with amino acids, the Maillard reaction .
Glucose can be used as a substrate for bacterial fermentation , for example with Clostridium thermoaceticum for the production of acetic acid , with Penicillium notatum for the production of araboascorbic acid , with Rhizopus delemar for the production of fumaric acid , with Aspergillus niger for the production of gluconic acid , with Candida brumptii for the production of isocitric acid with Aspergillus terreus for the production of itaconic acid , with Pseudomonas fluorescens for the production of 2-keto gluconic acid , with Gluconobacter suboxydans for the preparation of 5-ketogluconic acid , with Aspergillus oryzae for the preparation of kojic acid with Lactobacillus delbruckii for the preparation of lactic acid , with Lactobacillus brevis for preparing of malic acid , with Propionibacter shermanii for the production of propionic acid , with Pseudomonas aeruginosa for the production of pyruvic acid and with Gluconobacter suboxydans for the production of tartaric acid .
The following chart gives a brief overview of important products that can be produced biotechnologically. The industrially interesting products or their preliminary stages are marked in bold :
The enzyme cellulase is used to produce glucose from cellulose for the production of ethanol ( cellulose-ethanol ) for use as biofuel .
biochemistry
Glucose is mainly produced by plants with the help of photosynthesis from sunlight, water and carbon dioxide and can be used by all living things as a source of energy and carbon. A large part of the glucose in plants and animals is not free, but in bound form, for example in the form of lactose or beet sugar (sucrose) , or in the form of polymers such as starch or cellulose, which in plants are reserve substances or components of the cell wall . These polymers are first broken down into glucose with the help of enzymes when animals, fungi and bacteria eat them . In humans, this sometimes happens when chewing using amylase, which is contained in saliva , and maltase . All living beings are also able to produce glucose themselves from certain starting materials if the need arises. Nerve cells , cells of the renal medulla and erythrocytes depend on glucose for their energy production. In the adult human there is about 18 g of glucose, of which about 4 g is in the blood. Approximately 180 to 220 g of glucose are formed in the liver of an adult human in 24 hours.
Precursors to other biomolecules
In living beings, glucose is converted into several other chemical compounds that are the starting product of various metabolic pathways. All other monosaccharides such as fructose (via the polyol route ), mannose (the epimer at position 2), galactose (the epimer at position 4), fucose , various uronic acids and the amino sugars are produced from glucose . In addition to phosphorylation to glucose-6-phosphate , which is part of glycolysis , glucose can initially be oxidized to glucono-1,5-lactone when it is broken down. In some bacteria, glucose serves as a building block in the biosynthesis of trehalose or dextran and in animals as a building block for glycogen. Glucose can also be converted into fructose by the bacterial xylose isomerase . In addition, all non-essential amino acids , sugar alcohols such as mannitol and sorbitol , fatty acids , cholesterol and nucleic acids are produced from the metabolic products of glucose . Finally, glucose is used as a building block in the glycosylation required for the function of many proteins and also in other glycosylated substances such as glycoproteins , glycolipids , peptidoglycans and glycosides . The glycosylation is catalyzed by glycosyltransferases and can be split off again by glycosidases .
admission
In humans, glucose ingested from food first binds to the sweet taste receptor on the tongue. This complex of the proteins T1R2 and T1R3 makes it possible to identify food sources containing glucose. Glucose comes mainly from food - around 300 g per day is produced by converting food, but it is also synthesized from other metabolites in the body's cells. Glucose is a component of many carbohydrates and can be split off from them with the help of certain enzymes . Glucosidases (a subgroup of glycosidases) first catalyze the hydrolysis of long-chain glucose-containing polysaccharides, with terminal glucose being removed. Disaccharides, in turn, are usually broken down into glucose by special glycosidases . The names of the degrading enzymes are often derived from the respective poly- and disaccharide; For example, there are amylases (from amylose , part of starch ), cellulases (from cellulose ), chitinases (from chitin ) and more for the breakdown of polysaccharide chains ; furthermore for the cleavage of disaccharides the lactase , saccharase , trehalase and others. About 70 genes are known in humans that code for glycosidases. They have functions in the digestion and in the breakdown of glycogen, sphingolipids , mucopolysaccharides and poly (ADP-ribose) . In humans, polysaccharides containing glucose are partly broken down during chewing by the amylase contained in saliva , as well as by the maltase , lactase and saccharase in the brush border of the small intestine .
In order to get into or out of cells and cell compartments via the cell membrane , the glucose needs special transport proteins from the major facilitator superfamily . In the small intestine (more precisely in the jejunum ), glucose is taken up into the intestinal epithelial cells with the aid of the glucose transporter via a secondary active transport mechanism called sodium ion-glucose symport ( sodium / glucose cotransporter 1 ) . It is passed on on the basolateral side of the intestinal epithelial cell via the glucose transporter GLUT2 , as is its uptake in hepatocytes , kidney cells , β cells of the islets of Langerhans, neurons, astrocytes and tanycytes . Glucose reaches the liver via the portal vein and is stored there in the form of glycogen. In the liver cell it is phosphorylated to glucose-6-phosphate by the glucokinase at position 6 ; so she can no longer leave the cell. With the help of glucose-6-phosphatase , glucose-6-phosphate is converted back into glucose if necessary, exclusively in the liver, so that it is available to maintain a sufficient blood concentration. In other cells, uptake occurs through passive transport through one of the 14 GLUT proteins. In the other cell types , phosphorylation is carried out by a hexokinase , whereupon glucose can no longer diffuse out of the cell.
GLUT1 is formed by most cell types and has special meaning for nerve cells and the pancreas contained β-cells . GLUT3 is often formed by nerve cells. Glucose is absorbed from the bloodstream by muscle cells (skeletal muscles and heart muscle) and fat cells via GLUT4 . GLUT14 is only produced in testes . Excess glucose is broken down and converted into fatty acids, which are stored as triacylglyceride . In the kidneys, glucose is absorbed from the urine via SGLT1 and SGLT2 in the apical cell membranes and passed on via GLUT2 in the basolateral cell membranes. About 90% of the kidney's glucose absorption occurs via SGLT2 and about 3% via SGLT1.
biosynthesis
The metabolic pathway for building glucose from small molecules of two to four carbon atoms, which ends in the glucose molecule with six carbon atoms, is called gluconeogenesis and occurs in all living things. The smaller starting materials are the result of other metabolic pathways and, in plants, ultimately come from the assimilation of carbon dioxide . Ultimately, almost all biomolecules come from the assimilation of carbon dioxide in plants during photosynthesis. The free energy of the formation of α-D-glucose is 917.2 kilojoules per mole. In humans, gluconeogenesis takes place in the liver and kidneys , but also in other cell types .
The breakdown of glycogen is known as glycogenolysis, the breakdown of starch as starch breakdown. About 150 g of glycogen are stored in the liver and about 250 g in the skeletal muscles . The glucose released in muscle cells when the glycogen is broken down cannot be released into the bloodstream, however, since glucose is phosphorylated by the hexokinase, no glucose-6-phosphatase is formed to remove the phosphate group and for glucose-6-phosphate in contrast to glucose no transport protein exists. Through gluconeogenesis, the organism can build up glucose from other metabolic products, including lactate or certain amino acids, while consuming energy. The tubular cells of the kidneys can also produce glucose.
Dismantling
Dextrose is broken down in the metabolism via glycolysis and the pentose phosphate pathway. Glycolysis is used by all living organisms with small variations, and all organisms generate energy from the breakdown of monosaccharides. In the further course of the metabolism, it can be completely broken down into water and carbon dioxide via oxidative decarboxylation , the citric acid cycle and the respiratory chain . If there is not enough oxygen available for this, glucose is broken down anaerobically in animals through lactic acid fermentation, down to lactate, and less energy is released. In mammals, lactate from the muscles reaches the liver via the bloodstream, where gluconeogenesis takes place ( Cori cycle ). Other forms of fermentation occur in other living things . If there is a high supply of glucose, the metabolite acetyl-CoA is also used for fatty acid synthesis . Glucose also replenishes the body's glycogen stores, which are mainly found in the liver and skeletal muscles. These processes are regulated by hormones.
The bacterium Escherichia coli can grow on nutrient media that have glucose as the only carbon source. In some bacteria and - in a modified form - also in archaea , glucose is broken down via the Entner-Doudoroff path .
Tumor cells often grow comparatively quickly and consume an above-average amount of glucose via glycolysis, which leads to the formation of lactate, the end product of fermentation in mammals, even in the presence of oxygen . This effect is known as the Warburg effect . Various SGLT and GLUT are increasingly formed for the increased uptake of glucose in tumors . In yeast, at high glucose concentrations, even in the presence of oxygen (usually leads to breathing , but not to fermentation), ethanol is produced by fermentation. This effect is known as the Crabtree effect .
Calorific value

The physiological calorific value of glucose is, depending on the source, 16.2 kilojoules per gram or 15.7 kJ / g (3.74 kcal / g). The high availability of carbohydrates through plant biomass has led to a variety of methods during evolution , especially of microorganisms , to utilize the energy and carbon storage glucose. There are differences in the end product that can no longer be used for energy generation. Here, the presence of individual genes and their gene products, the enzymes, decide which reactions are possible (see figure). The metabolic pathway of glycolysis is used by almost all living things. An essential difference in this way is the production of NADP as a reducing agent for anabolism , which would otherwise have to be produced indirectly.
Glucose concentrations
The glucose in the blood is called blood sugar . The blood sugar level is regulated by glucose-binding nerve cells in the hypothalamus . In addition, glucose in the brain binds to glucose receptors in the reward center in the nucleus accumbens . The binding of glucose to the sweet taste receptor on the tongue triggers the release of various hormones of the energy metabolism with and without ingestion of glucose, which lead to increased absorption in cells and a lowering of the blood sugar level. On the other hand, sweeteners do not lower blood sugar levels.
The fasted blood sugar content of a healthy person is, i. H. after overnight fasting , about 70 to 100 mg / dl blood (4 to 5.5 mM ). In the blood plasma , the values measured are around 10–15% higher. In addition, the values in arterial blood are higher than the concentrations in venous blood, since glucose is absorbed into the tissue during the passage of the capillary bed. In capillary blood, too, which is often used to determine blood sugar levels, the values are sometimes higher than in venous blood. The glucose content of the blood is regulated by the hormones insulin , incretin and glucagon : insulin lowers the glucose level, glucagon increases it. Furthermore, the hormones adrenaline , thyroxine , glucocorticoids , somatotropin and adrenocorticotropin lead to an increase in the glucose level. In addition, there is also a hormone-independent regulation, which is called glucose autoregulation . After eating, the blood sugar concentration increases. Values above 180 mg / dl in venous whole blood are pathological and are called hyperglycaemia , values below 40 mg / dl are called hypoglycaemia .
When used, glucose is released into the bloodstream by the glucose-6-phosphatase from glucose-6-phosphate as well as from glycogen from the liver and kidneys, whereby a homeostasis of the blood glucose concentration is achieved. In ruminants , the blood glucose concentration is lower (60 mg / dL in cattle and 40 mg / dL in sheep) because the carbohydrates are converted more into short-chain fatty acids by the intestinal flora .
In the brain , which relies on glucose as the main energy supplier , the glucose concentration is normally 4 to 6 mM (5 mM corresponds to 90 mg / dL), but drops to 2 to 3 mM during fasting . Low glucose concentrations in the brain have a negative effect on the ability to think, self-control and will . Below 1 mM, confusion occurs, and below 1 mM, coma occurs.
The glycemic index is an indicator of the rate of absorption and conversion to blood glucose from ingested carbohydrates and is determined as the integral of the blood glucose level after ingestion compared to glucose (ingested glucose is defined as 100). The clinical significance of the glycemic index is controversial because foods with a high fat content slow the absorption of glucose and thus lower the glycemic index, such as ice cream. An alternative measure with the same problem is the insulin index , measured as the influence of the intake of carbohydrates on the insulin level in the blood. The glycemic load is an indicator of the amount of glucose that is added to the blood stream after ingestion of carbohydrates, and is based on the glycemic index and the amount of ingested food.
Pathobiochemistry
Autoimmune diabetes
In the course of an autoimmune reaction against the β cells in the islets of Langerhans in the pancreas , the β cells are killed, which means that the hormone insulin is no longer produced. This gives rise to type I diabetes mellitus (autoimmune diabetes). Insulin or analogs must then be administered regularly by subcutaneous injection, depending on the result of a measurement with a blood glucose meter .
Acquired Diabetes
Dysregulation of the glucose level is referred to as intermediate hyperglycaemia and, in more severe forms, as diabetes mellitus type II (synonym for acquired diabetes , insulin resistance ). A repeated or permanent high blood sugar level usually indicates diabetes mellitus. Depending on the severity, oral antidiabetic drugs can be used. Regular exercise and avoidance of obesity reduce the risk of type II diabetes mellitus, and exercise is recommended for the treatment of type II diabetes mellitus.
Overweight and fatty liver
Furthermore, an increased intake of glucose leads to obesity and, as a result, in some cases to the metabolic syndrome with non-alcoholic fatty liver hepatitis , but not the consumption of glucose in the context of a normal calorie intake.
Analytics
Specifically, when a glucose molecule to be detected at a particular position in a larger molecule, a nuclear magnetic resonance spectroscopy , an X-ray crystal structure analysis or a lectin - immunostaining with a concanavalin A - reporter enzyme - conjugate (only binds glucose or mannose) is performed.
Classic qualitative detection reactions
These reactions are only of historical significance:
Fehling's reaction
The Fehling sample is a classic proof of aldoses. Due to the mutarotation , a small proportion of glucose is always present as an open-chain aldehyde. By adding the Fehling's reagents (Fehling (I) solution and Fehling (II) solution), the aldehyde group is oxidized to the carboxylic acid, while the Cu 2+ tartrate complex is reduced to Cu + and becomes a brick-red precipitate (Cu 2 O ) fails.
Great reaction
With the Tollens sample , after adding ammoniacal AgNO 3 to the sample solution, Ag + is reduced from glucose to elemental silver.
Barfoedsche sample
In the Barfoed test , a mixture of dissolved copper acetate , sodium acetate and acetic acid is mixed with the solution of the sugar to be examined and heated in a water bath for a few minutes. Glucose and other monosaccharides quickly develop a reddish color and reddish brown copper (I) oxide (Cu 2 O).
Nylander's reagent
As a reducing sugar , glucose reacts with Nylander's reagent .
Further evidence
When a dilute potassium hydroxide solution with glucose is heated to 100 ° C, a strong reddish browning and a caramel-like odor develop. Concentrated sulfuric acid dissolves dry glucose without blackening at room temperature, with the formation of sugar sulfuric acid . In solution with yeast , alcoholic fermentation immediately produces carbon dioxide in the ratio of 2.0454 molecules of glucose to one molecule of CO 2 . Glucose forms a black mass with tin chloride . In ammoniacal silver solution , glucose (like lactose and dextrin ) leads to a deposition of silver without the formation of a silver mirror . In ammoniacal lead acetate solution , white lead glycosate forms in the presence of glucose , which becomes less soluble and turns brown when cooked. In ammoniacal copper solution, yellow copper oxide hydrate is formed with glucose at room temperature , while boiling, on the other hand, forms red copper oxide (with dextrin as well, except for ammoniacal copper acetate solution ). With Hager's reagent , glucose forms mercury oxide when cooked . With an alkaline bismuth solution , elemental, black-brown bismuth is deposited with glucose. Glucose boiled in ammonium molybdate solution turns the solution blue. A solution with indigo carmine and sodium carbonate discolors when boiled with glucose.
Instrumental quantitative determination
Refractometry and polarimetry
In concentrated solutions of glucose with a small proportion of other carbohydrates, their concentration can be determined with a polarimeter . In the case of sugar mixtures, the concentration can be determined with a refractometer , for example when determining Oechsle in the course of the production of wine .
Photometric-enzymatic method in solution
The enzyme glucose oxidase (GOx) converts glucose into gluconic acid and hydrogen peroxide using oxygen . Another enzyme, peroxidase, catalyzes a chromogenic reaction (Trinder reaction) of phenol with 4-aminoantipyrine to form a purple color.
Photometric test strip method
The test strip method uses the aforementioned enzymatic conversion of glucose to gluconic acid with the formation of hydrogen peroxide. The reagents are immobilized on a polymer matrix, the so-called test strip, which takes on a more or less strong color. This can be read out reflectometrically with the aid of an LED-based handheld photometer at 510 nm. This enables routine blood glucose testing by laypeople. In addition to the reaction of phenol with 4-aminoantipyrine, new chromogenic reactions have been developed which allow photometry at higher wavelengths (550 nm, 750 nm).
Amperometric glucose sensors
The electroanalysis of glucose is also based on the enzymatic conversion mentioned above. The hydrogen peroxide produced can be quantified amperometrically by anodic oxidation at a potential of 600 mV. The GOx is immobilized on the electrode surface or in a membrane arranged close to the electrode. In addition to the classic noble metals such as platinum or gold, carbon nanotube electrodes have recently been used more and more frequently as electrodes. B. were doped with boron. Cu-CuO nanowires are also used as enzyme-free amperometric electrodes. A detection limit of 50 µmol / L was thus achieved. A particularly promising method is so-called "enzyme wiring". The electron flowing during the oxidation is diverted directly from the enzyme to the electrode via a molecular wire.
Other sensory methods
A variety of other chemical sensors exist for glucose. In view of the importance of the analysis of glucose in the biosciences, numerous optical probes for saccharides have been developed that are based on the use of boronic acids and are particularly suitable for intracellular, sensory applications where other (optical) methods are not at all or only to a limited extent can be used. In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups of the sugar, there are other probe concepts classified according to functional mechanisms that use selective glucose-binding proteins (e.g. Concanavalin A) as receptors. In addition, methods have been developed which measure the glucose concentration indirectly via the concentration of metabolized products, e.g. B. the consumption of oxygen with the help of fluorescent optical sensors. Finally, there are enzyme-based concepts that use the intrinsic absorbance or fluorescence of (fluorescence-marked) enzymes as information carriers.
Copper iodometry
Glucose can be made by copper - Iodometrie be quantified.
Chromatographic Process
Especially for the analysis of complex mixtures containing glucose, such as. B. in honey , chromatographic processes such as high-performance liquid chromatography and gas chromatography are used today , often in combination with mass spectrometry . Taking into account the isotope ratios , this analysis can also reliably detect adulteration of honey caused by added sugar. Derivatizations using silylation reagents have proven to be advantageous . In this way, the proportions of di- and trisaccharides can also be quantified.
In vivo analysis
The glucose uptake in cells of organisms is examined with 2-deoxy-D-glucose or fluorodeoxyglucose . ( 18 F) -Fluorodeoxyglucose is used in oncology and neurology as a tracer in positron emission tomography , where it is by far the most widely used diagnostic tool.
literature
- Jochen Lehmann: Carbohydrates. Chemistry and biology. 2., rework. and exp. Edition. Thieme, Stuttgart / New York 1996, ISBN 3-13-532902-X .
- Hans Vogel: tables of sugars and their derivatives. Springer-Verlag, 2013, ISBN 978-3-642-47764-5
- Günther Wolff: The sugar metabolism - a biographical study. In: Medical monthly . Volume 12, 1958, pp. 766-774 and 838-846.
Web links
Individual evidence
- ↑ Entry on GLUCOSE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d Entry for CAS no. 50-99-7 in the GESTIS substance database of the IFA , accessed on September 18, 2014(JavaScript required) .
- ^ Alfred Töpel: Chemistry and Physics of Milk: Natural Material - Raw Material - Food . Behr's Verlag DE, 2004, ISBN 3-89947-131-8 , p. 101 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-268.
- ↑ a b c Entry on d-glucose. In: Römpp Online . Georg Thieme Verlag, accessed on May 31, 2013.
- ↑ Glucose data sheet (PDF) from Carl Roth , accessed on August 24, 2010.
- ↑ Thénard, Gay-Lussac, Biot and Dumas: Report sur un mémoire de M. Péligiot, intitulé: Recherches sur la nature et les propriétés chimiques des sucres . In: Comptes rendus (1838), Volume 7, pp. 106-113.
- ↑ Abraham J. Domb, Joseph Kost, David Wiseman: Handbook of Biodegradable Polymers . CRC Press, 1998. ISBN 978-1-4200-4936-7 . P. 275.
- ↑ Kenji Kamide: Cellulose and Cellulose Derivatives . Elsevier, 2005, ISBN 978-0-080-45444-3 , p. 1.
- ^ WHO Model List of Essential Medicines. In: World Health Organization. October 2013, archived from the original on April 23, 2014 ; accessed on April 22, 2014 (English).
- ^ A b Benjamin Caballero, Paul Finglas, Fidel Toldrá: Encyclopedia of Food and Health . Academic Press (2016). ISBN 9780123849533 , Volume 3, pp. 239, 608.
- ↑ Marggraf: Experiences chimiques faites dans le dessein de tirer un veritable sucre de diverse plantes, qui croissent dans nos contrées. 'Chemical experiments with the intention of extracting real sugar from various plants growing in our lands'. In: Histoire de l'académie royale des sciences et belles-lettres de Berlin (1747), p. 90: Les raisins secs, etant humectés d'une petite quantité d'eau, de maniere qu'ils mollissent, peuvent alors etre pilés , & le suc qu'on en exprime, etant depuré & épaissi, fournira une espece de Sucre. 'Raisins, moistened with a little water so that they soften, can be squeezed, and the squeezed juice, cleaned and thickened, makes a sort of sugar.'
- ^ Aaron J. Ihde: The Development of Modern Chemistry. Harper and Row, New York 1964.
- ↑ a b John F. Robyt: Essentials of Carbohydrate Chemistry. (= Springer Advanced Texts in Chemistry ) 1998, ISBN 0-387-94951-8 .
- ↑ Tentative Rules for Carbohydrate Nomenclature Journal of Biological Chemistry No. 247, p. 613, 1972.
- ↑ John F. Robyt: Essentials of Carbohydrate Chemistry. Springer Science & Business Media, 2012, ISBN 978-1-461-21622-3 . P. 7.
- ↑ MA Rosanoff: On Fischer's classification of stereo-isomers. In: Journal of the American Chemical Society . 28, 1906, p. 114, doi : 10.1021 / ja01967a014 .
- ^ Emil Fischer - Biographical. In: nobelprize.org. July 15, 1919, accessed June 4, 2018 .
- ↑ Bert Fraser-Reid: van 't Hoff's glucose . In: Chem. Eng. News , 1999, Volume 77, Issue 39, p. 8; doi: 10.1021 / cen-v077n039.p008 .
- ^ IUPAC: Nomenclature of Carbohydrates (Recommendations 1996) .
- ↑ Otto Meyerhof - Facts. In: nobelprize.org. July 15, 2018, accessed July 15, 2018 .
- ↑ Hans von Euler-Chelpin - Facts. In: nobelprize.org. Accessed September 3, 2018 .
- ^ Arthur Harden - Facts. In: nobelprize.org. June 17, 1940, accessed September 3, 2018 .
- ↑ Bernardo Houssay - Facts. In: nobelprize.org. September 21, 1971, accessed July 15, 2018 .
- ^ Carl Cori - Facts. In: nobelprize.org. October 20, 1984, accessed July 15, 2018 .
- ^ Gerty Cori - Facts. In: nobelprize.org. October 26, 1957, accessed July 15, 2018 .
- ↑ Luis Leloir - Facts. In: nobelprize.org. July 15, 2018, accessed July 15, 2018 .
- ↑ Fred W. Schenck: Glucose and Glucose-Containing Syrups . In: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim, 2006. doi : 10.1002 / 14356007.a12_457.pub2 .
- ↑ Patrick F. Fox: Advanced Dairy Chemistry Volume 3: Lactose, water, salts and vitamins , Springer, 1992. Volume 3, ISBN 9780412630200 . P. 316.
- ^ Benjamin Caballero, Paul Finglas, Fidel Toldrá: Encyclopedia of Food and Health . Academic Press (2016). ISBN 9780123849533 , Volume 1, p. 76.
- ↑ HF Bunn, PJ Higgins: Reaction of monosaccharides with proteins: possible evolutionary significance . In: Science . 213, No. 4504, 1981, pp. 222-224. doi : 10.1126 / science.12192669 .
- ↑ Jeremy M. Berg: Stryer Biochemistry. Springer-Verlag, 2017, ISBN 978-3-662-54620-8 , p. 531.
- ^ Reginald H. Garrett: Biochemistry. Cengage Learning, 2012, ISBN 978-1-133-10629-6 . Pp. 194, 199.
- ↑ Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0470-57095-1 . P. 363.
- ^ Albert L. Lehninger, Biochemistry, 6th printing , Worth Publishers Inc. 1972, ISBN 0-87901-009-6 , p. 228.
- ^ A b c Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry. Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , p. 27.
- ↑ Eusebio Juaristi, Gabriel Cuevas: The anomeric Effect CRC Press, 1995. ISBN 0-8493-8941-0 . Pp. 9-10.
- ↑ a b Manfred Hesse, Herbert Meier, Bernd Zeeh, Stefan Bienz, Laurent Bigler, Thomas Fox: Spectroscopic methods in organic chemistry . 8., revised. Edition. Georg Thieme, 2011, ISBN 978-3-13-160038-7 , p. 34 .
- ^ Food Composition Databases Show Foods List. In: ndb.nal.usda.gov. Retrieved August 25, 2018 .
- ^ Oregon State University : Sugar ( July 18, 2011 memento on the Internet Archive ), accessed June 28, 2018.
- ↑ a b c d e f g h i P J Fellows: Food Processing Technology. Woodhead Publishing, 2016, ISBN 978-0-081-00523-1 , p. 197.
- ↑ a b Thomas Becker, Dietmar Breithaupt, Horst Werner Doelle, Armin Fiechter, Günther Schlegel, Sakayu Shimizu, Hideaki Yamada: Biotechnology , in: Ullmann's Encyclopedia of Industrial Chemistry , 7th edition, Wiley-VCH, 2011. ISBN 978-3- 527-32943-4 . Volume 6, p. 48.
- ↑ Starch sugar . In: Merck's Warenlexikon . 3rd ed. 1884 ff., P. 457 f.
- ^ A b Alan Davidson: The Oxford Companion to Food. OUP Oxford, 2014, ISBN 978-0-191-04072-6 , p. 527.
- ↑ a b c The Amylase Research Society of Japan: Handbook of Amylases and Related Enzymes. Elsevier, 2014, ISBN 978-1-483-29939-6 , p. 195.
- ↑ GB Madsen, BE Norman, S. Slott: A New, Heat Stable Bacterial Amylase and its Use in High Temperature Liquefaction . In: Starch (1973), Volume 25, Issue 9, doi : 10.1002 / star.19730250906 . Pp. 304-308.
- ^ BE Norman: A Novel Debranching Enzyme for Application in the Glucose Syrup Industry. In: Starch - strength . 34, 1982, p. 340, doi : 10.1002 / star.19820341005 .
- ↑ James N. BeMiller, Roy L. Whistler ,: Starch: Chemistry and Technology (= Food Science and Technology), 3rd. Edition, Academic Press, New York 2009, ISBN 008092655X .
- ↑ High Fructose Corn Syrup: Questions and Answers. US Food and Drug Administration November 5, 2014, archived from the original January 25, 2018 ; accessed on December 18, 2017 (English).
- ↑ Kevin Pang: Mexican Coke a hit in US ( Memento June 29, 2011 in the Internet Archive ) In: Seattle Times October 29, 2004.
- ↑ Steve T. Beckett: Beckett's Industrial Chocolate Manufacture and Use. John Wiley & Sons, 2017, ISBN 978-1-118-78014-5 , p. 82.
- ^ Hans-Dieter Belitz , Werner Grosch , Peter Schieberle : Food chemistry . Springer, Berlin 2009. ISBN 978-3-540-69935-4 . Pp. 270-289.
- ^ Nathan Myhrvold , Chris Young, Maxime Bilet: Modernist Cuisine: The Art and Science of Cooking . The Cooking Lab 2011. ISBN 978-0-9827610-0-7 . Volume 3, pp. 89ff.
- ↑ James A. Kent: Riegel's Handbook of Industrial Chemistry. Springer Science & Business Media, 2013, ISBN 978-1-475-76431-4 , p. 938.
- ↑ Ashok Pandey: Industrial Biorefineries and White Biotechnology. Elsevier, 2015, ISBN 978-0-444-63464-1 , p. 488.
- ^ A b c Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry. Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , p. 195.
- ↑ a b c d e U. Satyanarayana: Biochemistry. Elsevier Health Sciences, 2014, ISBN 978-8-131-23713-7 . P. 674.
- ^ DH Wasserman: Four grams of glucose. In: American Journal of Physiology - Endocrinology and Metabolism. Volume 296, number 1, January 2009, pp. E11-E21, doi : 10.1152 / ajpendo.90563.2008 , PMID 18840763 , PMC 2636990 (free full text).
- ↑ a b c d e f g Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry. Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , pp. 199, 200.
- ^ A b Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry. Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , p. 214.
- ↑ Essentials of Glycobiology , Ajit Varki (ed.), 2nd. Edition, Cold Spring Harbor Laboratories Press ,, ISBN 978-0-87969-770-9 . Archived from the original on December 6, 2016.
- ^ Peter C. Heinrich: Löffler / Petrides Biochemistry and Pathobiochemistry . Springer-Verlag, 2014, ISBN 978-3-642-17972-3 , p. 404.
- ↑ Search result UniProt .
- ↑ Search result UniProt .
- ↑ Harold A. Harper: Medical Biochemistry. Springer-Verlag, 2013, ISBN 978-3-662-22150-1 , p. 641.
- ↑ AM Navale, AN Paranjape: Glucose transporters: physiological and pathological roles. In: Biophysical Reviews . Volume 8, number 1, March 2016, pp. 5-9, doi : 10.1007 / s12551-015-0186-2 , PMID 28510148 , PMC 5425736 (free full text).
- ↑ B. Thorens: GLUT2, glucose sensing and glucose homeostasis. In: Diabetologia . Volume 58, Number 2, February 2015, pp. 221-232, doi : 10.1007 / s00125-014-3451-1 , PMID 25421524 .
- ^ RC Bonadonna, S. Del Prato, E. Bonora, MP Saccomani, G. Gulli, A. Natali, S. Frascerra, N. Pecori, E. Ferrannini, D. Bier, C. Cobelli, RA DeFronzo: Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. In: Diabetes. Volume 45, Number 7, July 1996, pp. 915-925, PMID 8666143 .
- ↑ S. Huang, MP Czech: The GLUT4 glucose transporter. In: Cell Metabolism . Volume 5, Number 4, April 2007, pp. 237-252, doi : 10.1016 / j.cmet.2007.03.006 , PMID 17403369 .
- ^ R. Govers: Cellular regulation of glucose uptake by glucose transporter GLUT4. In: Advances in Clinical Chemistry . Volume 66, 2014, pp. 173-240, PMID 25344989 .
- ↑ C. Ghezzi, DD Loo, EM Wright: Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. In: Diabetologia . [Electronic publication before going to press] August 2018, doi : 10.1007 / s00125-018-4656-5 , PMID 30132032 .
- ↑ SB Poulsen, RA Fenton, T. Rieg: Sodium-glucose cotransport. In: Current opinion in nephrology and hypertension. Volume 24, number 5, September 2015, pp. 463-469, doi : 10.1097 / MNH.0000000000000152 , PMID 26125647 , PMC 5364028 (free full text).
- ↑ Donald Voet, Judith G. Voet: Biochemistry, 4th Edition . John Wiley & Sons, 2010, ISBN 978-0470-57095-1 . P. 359.
- ^ A b Donald Voet, Judith G. Voet: Biochemistry, 4th Edition. John Wiley & Sons, 2010, ISBN 978-0470-57095-1 . P. 59.
- ^ A b Leszek Szablewski: Glucose Homeostasis and Insulin Resistance. Bentham Science Publishers, 2011, ISBN 978-1-608-05189-2 , p. 46.
- ↑ AM Smith, SC Zeeman, SM Smith: Starch degradation. In: Annual Review of Plant Biology . Volume 56, 2005, pp. 73-98, doi : 10.1146 / annurev.arplant.56.032604.144257 , PMID 15862090 .
- ↑ MM-Adeva any tax, N. Pérez-Felpete, C. Fernandez-Fernandez, C. Donapetry-García, C. Pazos-García: Liver glucose metabolism in humans. In: Bioscience Reports . Volume 36, number 6, 12 2016, p. E00416, doi : 10.1042 / BSR20160385 , PMID 27707936 , PMC 5293555 (free full text).
- ^ H. Robert Horton, Laurence A. Moran, K. Gray Scrimgeour, Marc D. Perry, J. David Rawn and Carsten Biele (translators): Biochemie . Pearson Studies; 4th updated edition 2008; ISBN 978-3-8273-7312-0 ; Pp. 490-496.
- ^ A b Brian K. Hall: Strickberger's Evolution. Jones & Bartlett Publishers, 2013, ISBN 978-1-449-61484-3 , p. 164.
- ^ Reginald H. Garrett: Biochemistry. Cengage Learning, 2012, ISBN 978-1-133-10629-6 , p. 551.
- ↑ JG Jones: Hepatic glucose and lipid metabolism. In: Diabetologia . Volume 59, number 6, 06 2016, pp. 1098–1103, doi : 10.1007 / s00125-016-3940-5 , PMID 27048250 .
- ↑ Entner, N. and Doudoroff, M. (1952): Glucose and gluconic acid oxidation of Pseudomonas saccharophila . In: J Biol Chem . 196 (2); Pp. 853-862; PMID 12981024 ; PDF (free full text access).
- ^ A. Annibaldi, C. Widmann: Glucose metabolism in cancer cells. In: Current Opinion in Clinical Nutrition and Metabolic Care . Volume 13, Number 4, July 2010, pp. 466-470, doi : 10.1097 / MCO.0b013e32833a5577 , PMID 20473153 .
- ↑ XD Xu, SX Shao, HP Jiang, YW Cao, YH Wang, XC Yang, YL Wang, XS Wang, HT Niu: Warburg effect or reverse Warburg effect? A review of cancer metabolism. In: Oncology Research and Treatment . Volume 38, number 3, 2015, pp. 117-122, doi : 10.1159 / 000375435 , PMID 25792083 .
- ↑ L. Szablewski: Expression of glucose transporters in cancers. In: Biochimica et Biophysica Acta . Volume 1835, Number 2, April 2013, pp. 164-169, doi : 10.1016 / j.bbcan.2012.12.004 , PMID 23266512 .
- ↑ K. Adekola, ST Rosen, M. Shanmugam: Glucose transporters in cancer metabolism. In: Current Opinion in Oncology . Volume 24, Number 6, November 2012, pp. 650-654, doi : 10.1097 / CCO.0b013e328356da72 , PMID 22913968 .
- ^ RH De Deken: The Crabtree effect: a regulatory system in yeast. In: Journal of General Microbiology . Volume 44, Number 2, August 1966, pp. 149-156, doi : 10.1099 / 00221287-44-2-149 , PMID 5969497 .
- ↑ E. de Alteriis, F. Cartenì, P. Parascandola, J. Serpa, S. Mazzoleni: Revisiting the Crabtree / Warburg effect in a dynamic perspective: a fitness advantage against sugar-induced cell death. In: Cell Cycle . Volume 17, number 6, 2018, pp. 688-701, doi : 10.1080 / 15384101.2018.1442622 , PMID 29509056 , PMC 5969562 (free full text).
- ↑ Georg Schwedt Zuckersüße chemistry. John Wiley & Sons, 2012, ISBN 978-3-527-66001-8 , p. 100.
- ^ Schmidt, Lang: Physiologie des Menschen, 30th edition. Springer Verlag, 2007, p. 907.
- ↑ T. Dandekar, S. Schuster, B. Snel, M. Huynen, P. Bork: Pathway alignment: application to the comparative analysis of glycolytic enzymes. In: Biochem. J. 343 Pt 1, 1999, pp. 115-124 ( PMID 10493919 ; PMC 1220531 (free full text)).
- ↑ a b c L. L. Koekkoek, JD Mul, SE la Fleur: Glucose-Sensing in the Reward System. In: Frontiers in Neuroscience . Volume 11, 2017, p. 716, doi : 10.3389 / fnins.2017.00716 , PMID 29311793 , PMC 5742113 (free full text).
- ↑ a b R. M. Tucker, SY Tan: Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review. In: Physiology & Behavior . Volume 182, December 2017, pp. 17-26, doi : 10.1016 / j.physbeh.2017.09.016 , PMID 28939430 .
- ^ SE La Fleur, E. Fliers, A. Kalsbeek: Neuroscience of glucose homeostasis. In: Handbook of Clinical Neurology . Volume 126, 2014, pp. 341-351, doi : 10.1016 / B978-0-444-53480-4.00026-6 , PMID 25410233 .
- ^ PH Bisschop, E. Fliers, A. Kalsbeek: Autonomic regulation of hepatic glucose production. In: Comprehensive Physiology . Volume 5, Number 1, January 2015, pp. 147-165, doi : 10.1002 / cphy.c140009 , PMID 25589267 .
- ^ WA Scherbaum, BM Lobnig, In: Hans-Peter Wolff, Thomas R. Weihrauch: Internal therapy 2006, 2007. 16th edition. Elsevier, Munich 2006, ISBN 3-437-23182-0 , pp. 927, 985.
- ↑ Harold A. Harper: Medical Biochemistry. Springer-Verlag, 2013, ISBN 978-3-662-22150-1 , p. 294.
- ↑ a b c Donard Dwyer: Glucose Metabolism in the Brain. Academic Press, 2002, ISBN 978-0-123-66852-3 , p. XIII.
- ↑ SH Fairclough, K. Houston: A metabolic measure of mental effort. In: Biological Psychology . Volume 66, Number 2, April 2004, pp. 177-190, doi : 10.1016 / j.biopsycho.2003.10.001 , PMID 15041139 .
- ↑ MT Gailliot, RF Baumeister: The physiology of willpower: linking blood glucose to self-control. In: Personality and social psychology review: an official journal of the Society for Personality and Social Psychology, Inc. Volume 11, Number 4, November 2007, pp. 303-327, doi : 10.1177 / 1088868307303030 , PMID 18453466 .
- ^ A b Richard A. Harvey, Denise R. Ferrier: Biochemistry. 5th Edition, Lippincott Williams & Wilkins, 2011, ISBN 978-1-608-31412-6 , p. 366.
- ↑ a b U. Satyanarayana: Biochemistry. Elsevier Health Sciences, 2014, ISBN 978-8-131-23713-7 , p. 508.
- ↑ SH Holt, JC Miller, P. Petocz: An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. In: The American Journal of Clinical Nutrition . Volume 66, issue 5, November 1997, pp. 1264-1276, doi : 10.1093 / ajcn / 66.5.1264 , PMID 9356547 .
- ^ P. Concannon, SS Rich, GT Nepom: Genetics of type 1A diabetes. In: The New England Journal of Medicine . Volume 360, Number 16, April 2009, pp. 1646-1654, doi : 10.1056 / NEJMra0808284 , PMID 19369670 .
- ↑ R. Goyal, I. Jialal: Glucose Intolerance. In: StatPearls [Internet]. Treasure Island (FL), 2018. PMID 29763085 .
- ↑ R. Buresh: Exercise and glucose control. In: The Journal of Sports Medicine and Physical Fitness . Volume 54, Number 4, August 2014, pp. 373-382, PMID 25034542 .
- ↑ H. Yki-Järvinen: Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. In: The Lancet . Diabetes & endocrinology. Volume 2, number 11, November 2014, pp. 901-910, doi : 10.1016 / S2213-8587 (14) 70032-4 , PMID 24731669 .
- ↑ H. Fehling: Quantitative determination of sugar in urine. In: Archives for Physiological Medicine (1848), Volume 7, pp. 64–73.
- ↑ B. Tollens: About ammoniacal silver solution as a reagent for aldehyde . In: Reports of the German Chemical Society . 15, 1882, pp. 1635-1639.
- ↑ C. Barfoed: About the detection of grape sugar in addition to dextrin and related bodies . In: Journal for Analytical Chemistry . 12, No. 1, 1873, p. 27. doi : 10.1007 / BF01462957 .
- ^ Emil Nylander: About alkaline bismuth solution as a reagent for glucose in urine , magazine for physiological chemistry . Volume 8, Issue 3, 1884, pp. 175-185 ( abstract ).
- ↑ a b c d e f g h i j k Georg Schwedt: Zuckersweet chemistry. John Wiley & Sons, 2012, ISBN 978-3-527-66001-8 , p. 102.
- ↑ P. Trinder: Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. In: Annals of Clinical Biochemistry . 6, 1969, pp. 24-27; doi: 10.1177 / 000456326900600108 .
- ↑ M. Mizoguchi, M. Ishiyama, M. Shiga, K. Sasamoto: Water-soluble chromogenic reagent for colorimetric detection of hydrogen peroxide - an alternative to 4-aminoantipyrine working at a long wavelength. In: Analytical Communications . 35, 1998, pp. 71-73; doi: 10.1039 / A709038B .
- ^ J. Wang: Electrochemical Glucose Biosensors. In: Chem. Rev. 108, 2008, pp. 814-825; PMID 18154363 .
- ↑ X. Chen, J. Chen, Ch. Deng, Ch. Xiao, Y. Yang, Z. Nie, S. Yao: Amperometric glucose biosensor based on boron-doped carbon nanotubes modified electrode. In: Talanta . 76, 2008, pp. 763-767; doi: 10.1016 / j.talanta.2008.04.023 ; PMID 18656655 .
- ↑ G. Wang, Y. Wei, W. Zhang, X. Zhang, B. Fang, L. Wang: Enzyme-free amperometric sensing of glucose using Cu-CuO nanowire composites. In: Microchimica Acta . 168, 2010, pp. 87-92; doi: 10.1007 / s00604-009-0260-1 .
- ^ TJ Ohara, R. Rajagopaian, A. Heller: "Wired" Enzyme Electrodes for Amperometric Determination of Glucose or Lactate in the Presence of Interfering Substances. In: Anal. Chem. 66, 1994, pp. 2451-2457; doi: 10.1021 / ac00087a008 ; PMID 8092486 .
- ↑ a b c S. M. Borisov, OS Wolfbeis: Optical Biosensors. In: Chem. Rev. 108, 2008, pp. 423-461; doi: 10.1021 / cr068105t ; PMID 18229952 .
- ↑ S. Ferri, K. Kojima, K. Sode: Review of glucose oxidases and glucose dehydrogenases: a bird's eye view of glucose sensing enzymes. In: Journal of Diabetes Science and Technology . Volume 5, number 5, September 2011, pp. 1068-1076, doi : 10.1177 / 193229681100500507 , PMID 22027299 , PMC 3208862 (free full text).
- ↑ HS Mader, OS Wolfbeis: Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. In: Microchimica Acta . 162, 2008, pp. 1-34; doi: 10.1007 / s00604-008-0947-8 .
- ↑ OS Wolfbeis, I. Oehme, N. Papkovskaya, I. Klimant: Sol-Gel based Glucose Biosensors Employing Optical Oxygen Transducers, and a Method for Compensating for Variable Oxygen Background. In: Biosensors & Bioelectronics . 15, 2000, pp. 69-76; doi: 10.1016 / S0956-5663 (99) 00073-1 .
- ↑ a b A. L. Galant, RC Kaufman, JD Wilson: Glucose: Detection and analysis. In: Food Chemistry . Volume 188, December 2015, pp. 149-160, doi : 10.1016 / j.foodchem.2015.04.071 , PMID 26041177 .
- ↑ ML Sanz, J. Sanz, I. Martínez-Castro: Gas chromatographic-mass spectrometric method for the qualitative and quantitative determination of disaccharides and trisaccharides in honey. In: Journal of Chromatography A 1059 (1-2), 2004, pp. 143-148; PMID 15628134 .
- ↑ [email protected]: GMD - Glucose (1MEOX) (5TMS) BP - InChI = 1S / C22H55NO6Si5 / c1-24-23-17-19 (26-31 (5.6) 7) 21 ( 28-33 (11.12) 13) 22 (29-34 (14.15) 16) 20 (27-32 (8.9) 10) 18-25-30 (2.3) 4 / h17.19- 22H. In: gmd.mpimp-golm.mpg.de. July 19, 2007, accessed June 4, 2018 .
- ^ AI Cabañero, JL Recio, M. Rupérez: Liquid chromatography coupled to isotope ratio mass spectrometry: a new perspective on honey adulteration detection. In: J Agric Food Chem . 54 (26), Dec 27, 2006, pp. 9719-9727; PMID 17177492 .
- ↑ M. Becker, F. Ler, T. Rosenau, A. Potthast: Ethoximation-silylation approach fiebnor mono- and disaccharide analysis and characterization of their identification parameters by GC / MS. In: Talanta . 115, 2013, pp. 642-651; PMID 24054643 .
- ^ Gesellschaft Deutscher Chemiker: Annexes to the position paper of the Nuclear Chemistry Section ( Memento from March 31, 2010 in the Internet Archive ), February 2000.
- ↑ Simone Maschauer, Olaf Prante: Sweetening Pharmaceutical Radiochemistry by 18 F-Fluoroglycosylation: A Short Review , in: BioMed Research International , Volume 2014, Article ID 214748; doi: 10.1155 / 2014/214748 ; PMID 24991541 ; PMC 4058687 (free full text, PDF).





