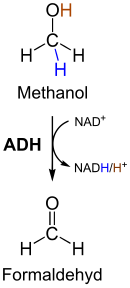Alcoholic fermentation
| Parent |
|
Glycolytic fermentation D- glucose metabolism |
| Gene Ontology |
|---|
| QuickGO |

The alcoholic fermentation is an enzymatic process in which carbohydrates , mainly glucose , under anoxic conditions to ethanol ( "potable alcohol") and carbon dioxide (degraded fermented be). Most microorganisms (microbes) capable of alcoholic fermentation only use this metabolic pathway temporarily to generate energy when the oxygen required for breathing is lacking.
The use of alcoholic fermentation by humans to produce alcoholic beverages already existed in prehistoric times. Nowadays, alcoholic fermentation also serves to convert biomass into ethanol as a secondary energy source .
Research history
Already in prehistoric times, hunters and gatherers produced alcoholic beverages. In 1815, the French chemist Joseph Louis Gay-Lussac set up the gross reaction equation for the breakdown of glucose into ethanol for the first time. Then different views developed about the process of fermentation. While in the 1830s Jöns Jakob Berzelius and Justus von Liebig attributed a catalyzing effect to certain substances with the "mechanistic theory of fermentation" , Charles Cagniard-Latour , Theodor Schwann and Friedrich Traugott Kützing believed , independently of one another, that living beings , namely yeasts , were responsible for this. In 1857, Louis Pasteur postulated the “vitalistic fermentation theory”, according to which alcoholic fermentation was only possible in connection with living cells.
This controversy was decided on January 11, 1897 by the chemist Eduard Buchner with a publication on the proof of alcoholic fermentation using cell-free yeast extract. He made the substance zymase - according to current knowledge a mixture of different enzymes - responsible for the conversion of sugar into ethanol and received the Nobel Prize in Chemistry in 1907 "for his biochemical investigations and the discovery of cell-free fermentation". Closer investigations by Arthur Harden and William John Young led to the discovery of a phosphorylated intermediate: the Harden-Young ester, known as fructose-1,6-bisphosphate . Together, Harden and Hans von Euler-Chelpin received the Nobel Prize for Chemistry in 1929 for their “research on sugar fermentation and the role of enzymes in this process”. After the partial reactions had been clarified bit by bit and schemes for the fermentation process had been drawn up, Otto Warburg identified the cofactor nicotinamide adenine dinucleotide (NADH) as an essential component of the fermentation process. As early as 1937, Erwin Negelein and Hans Joachim Wulff succeeded in crystallizing the fermentation enzyme alcohol dehydrogenase .
Other researchers who, after Buchner, contributed to the discovery of the enzyme chain in alcoholic fermentation were Carl Neuberg , Gustav Embden , Otto Fritz Meyerhof , Jakub Karol Parnas , Karl Lohmann as well as Gerty Cori and Carl Ferdinand Cori .
The enzymes from different species involved in fermentation have meanwhile been isolated and characterized biochemically ( pH optimum, temperature optimum, reaction speed, turnover rate). The crystal structure analysis gave a first insight into their molecular structure. There is knowledge about the reaction mechanisms. All in all, comparisons can be made between species. The deciphered genes , which contain the blueprints for these enzymes, provide information about their evolutionary origin and their possible original function.
The role in metabolism
Alcoholic fermentation is mainly carried out by sugar yeast ; in the absence of oxygen, it is used to generate energy. If oxygen is available, they break down sugar through cellular respiration and thus gain the energy they need for life. The sugar is completely oxidized to carbon dioxide and water through a long series of chemical, enzymatic (ie, catalyzed by enzymes) reactions ( glycolysis - citric acid cycle - respiratory chain ) while consuming oxygen. If no oxygen is available, the yeasts have an alternative way of producing energy in the alcoholic fermentation. However, compared to complete oxidation through cellular respiration, you can gain significantly less energy in the form of adenosine triphosphate (ATP) from glucose: When one molecule of glucose is broken down, 32 molecules of ATP can be obtained when it is fully oxidized, whereas alcoholic fermentation only produces 32 molecules of ATP two molecules of ATP. These two ATPs are obtained in glycolysis, the first step in the sequence of reactions in both cellular respiration and fermentation. The two further reaction steps of fermentation and thus ultimately ethanol production do not serve to generate energy, but to regenerate the cofactor NAD + , which is consumed in the enzymatic conversion of glycolysis. Since NAD is only available in limited quantities, the fermentation enzymes convert it from the reduced state (NADH) to the oxidized state (NAD + ) through oxidation with acetaldehyde ; the acetaldehyde is reduced to ethanol.
Yeasts are facultative anaerobes . When oxygen is available, glucose is metabolized aerobically, which is completely oxidized to carbon dioxide and water. In the absence of air, however, yeasts have to carry out alcoholic fermentation. Since this generates far less energy per molecule of converted glucose than aerobic respiration, the rate of glucose conversion increases sharply, which partially compensates for the lower ATP gain per molecule of converted glucose. This phenomenon is called the Pasteur effect . Because of the restricted energy production, yeasts grow and multiply far less rapidly in the absence of air than in the presence of air. In addition, the resulting ethanol acts as a cell poison.
If yeasts grow in a medium with a high sugar concentration and their cellular respiration enzymes are overloaded as a result, they carry out alcoholic fermentation even though there is sufficient oxygen. The yeasts constantly absorb the sugar and, in addition to cellular respiration, also utilize it through fermentation. This is the Crabtree effect .
In addition to types of yeast, some bacteria also carry out alcoholic fermentation. Sarcina ventriculi uses the same enzymatic path as yeast, while Zymomonas mobilis uses an alternative path. Likewise, low levels of ethanol formation in the presence of oxygen deficiency could be detected in various plants.
Biochemical basics
Enzymatic reactions
The first steps in alcoholic fermentation are glycolysis . For baker's yeast ( S. cerevisiae ) this is the Embden-Meyerhof-Parnas-Weg , while the bacterium Zymomonas mobilis uses the Entner-Doudoroff-Weg . Here, one molecule of D- glucose is converted into two molecules of pyruvate . In S. cerevisiae , two molecules of adenosine triphosphate (ATP) are formed from two molecules of adenosine diphosphate (ADP) and two phosphate residues (P i ) through substrate chain phosphorylation . In Z. mobilis only one molecule of ATP is formed. In addition, two molecules of NAD + (nicotinamide adenine dinucleotide) are reduced to two molecules of NADH in both ways .
In order for glycolysis to take place again, NAD + must be regenerated. This takes place under anaerobic conditions in the following fermentation reaction . One molecule of carbon dioxide is split off from each molecule of pyruvate by the enzyme pyruvate decarboxylase ( EC 4.1.1.1 ). Thiamine pyrophosphate , a relative of vitamin B 1 , and two magnesium ions serve as cofactors in this reaction . Pyruvate decarboxylase must not be confused with pyruvate dehydrogenase E1 ( EC 1.2.4.1 ) of the pyruvate dehydrogenase complex , which plays a central role in the aerobic breakdown of pyruvate.
The acetaldehyde produced in this step is very toxic for the organism and is immediately further converted in the following step. The catalyzing enzyme alcohol dehydrogenase ( EC 1.1.1.1 ) contains a zinc ion (Zn 2+ ), which polarizes the carbonyl group on acetaldehyde. This allows two electrons and one proton to be transferred from the NADH to the acetaldehyde, reducing it to ethanol and regenerating NAD + . Both glycolysis and the two subsequent reactions take place in the cytoplasm of the cell.
The net reaction equation for baker's yeast is:
Glucose, 2 adenosine diphosphate and 2 phosphate react to form 2 ethanol, 2 carbon dioxide and 2 adenosine triphosphate
The enzyme alcohol dehydrogenase produces ethanol by reducing acetaldehyde, but also catalyzes the reverse reaction . During the alcoholic fermentation, the reduction of acetaldehyde to ethanol takes place for the most part. The resulting ethanol is then released into the environment by the cells.
In contrast, the oxidation of ethanol to acetaldehyde takes place, for example, during the detoxification of ethanol in the liver . Acetaldehyde is poisonous and, along with fusel oils, is the main cause of headache and nausea after heavy alcohol consumption (the so-called " hangover "). Acetaldehyde is oxidized to acetic acid by the enzyme acetaldehyde dehydrogenase .
Byproducts
During the alcoholic fermentation by yeast, methanol and accompanying alcohols such as butanol , amyl alcohol and hexanol are produced as undesirable by-products . Their formation does not take place via the metabolic pathway described here, but for example via the breakdown of amino acids , methanol from the breakdown of pectins . In the body, the enzyme alcohol dehydrogenase oxidizes methanol to form toxic formaldehyde . If you drink alcohol with a high methanol content, the body produces a correspondingly large amount of formaldehyde, which is quickly converted into formic acid via aldehyde dehydrogenase . This is then metabolized into carbon dioxide and water. Since the breakdown of formic acid takes place more slowly than its formation, there is an accumulation of formic acid in the body and, as a result, metabolic acidosis , which in addition to damage to the optic nerve can ultimately lead to death. The known cases of methanol poisoning were almost exclusively those caused by the consumption of alcoholic beverages mixed with methanol.
regulation
Regulation, i.e. switching between aerobic cell respiration and anaerobic fermentation, is a current research topic. It is not possible to set up a general regulation scheme according to the system of 'throw the switch when there is a lack of X'. There are differences between individual yeast strains, as well as other processes in plants and bacteria. Researchers are in the process of clarifying the different reaction pathways. The oxygen content and the glucose level play a major role.
In addition, in S. cerevisiae, for example, there are two genes for the cytosolic enzyme alcohol dehydrogenase and thus two slightly different enzymes, ADH1 and ADH2 . Both enzymes can convert acetaldehyde into ethanol and vice versa. Due to small differences in their molecular structure, this happens at different speeds. ADH1 can build up ethanol faster, while ADH2 break down ethanol faster. The presence of the enzymes is regulated by transcription factors that control the reading of the respective gene. ADH1 for the ethanol build-up is always available. If the glucose level drops drastically, the enzyme ADH2 is produced, which can break down ethanol (if oxygen is present) to produce energy and thus keep the yeast alive. Yeast can build up ethanol when there is enough sugar, and later break down this ethanol itself when it is in dire need of energy. From an evolutionary point of view, it has an advantage: it poisons all food competitors with ethanol and then processes it again itself. The origin of the two genes for ADH1 and ADH2 is presumably due to the duplication of a common original gene . In other species there are also more than two alcohol dehydrogenases.
Energy balance
Since cell respiration with respiratory chain phosphorylation of ADP to ATP does not take place under anoxic conditions , the only energy source for yeast under these conditions is glycolysis with ATP formation through substrate phosphorylation . It supplies two molecules of ATP per molecule of glucose. In comparison, cell respiration would produce 32 molecules of ATP.
If the breakdown of glucose in pyruvate were to stop, the process would soon come to a standstill, as the NAD + consumption in glycolysis would lead to a NAD + deficiency. NAD + is only present in traces in the cell and has to be constantly regenerated. For this purpose, pyruvate is decarboxylated in the alcoholic fermentation and the acetaldehyde produced is reduced with NADH to ethanol, whereby NADH is oxidized to NAD + . If you take the entire reaction sequence from glucose to ethanol, no high-energy NADH is produced. If one looks at carbon, one third changes its oxidation number from 0 (in glucose) to +4 (carbon dioxide) and two thirds to −2 (ethanol). Alcoholic fermentation is thus a disproportionation as a special case of redox reactions .
The change in free energy under standard conditions, however, is pH 7 instead of 0, with alcoholic fermentation ΔG 0 '= - 218 kJ per mole of glucose, with cellular respiration - 2822 kJ per mole of glucose. The following standard conditions were agreed: temperature 25 ° C, pressure 1.013 bar, concentration of the substances involved in the reaction (reactants) 1 mol / L with the exception of that of water, for which 55.6 mol / L (pure water) has been agreed, and that of gases for which a concentration in solution equilibrium with a partial pressure of 1 bar in the gas phase has been agreed. In biological systems, however, the H + ion concentration is not agreed to be 1 mol / L, which is not tolerated by living beings, but 10 −7 mol / L, corresponding to pH 7. If the actual conditions deviate from these standard conditions, the amount of change in free energy is different; it can deviate considerably from the standard value. In living systems, standard conditions are usually not given and often change during the conversion of substances. The amount of change in free energy under standard conditions therefore only provides an indication of the energy released during chemical conversion in living beings.
Other substrates
In addition to glucose, other simple sugars can also be processed through glycolysis and thus through alcoholic fermentation. However, most yeasts have a special affinity for glucose (they are “glucophilic”), so that glucose is broken down preferentially during the alcoholic fermentation of grape must, which contains equal parts of glucose and fructose. If the finished wine is still sweet because not all of the sugar has been broken down into alcohol, most of the remaining sugar consists of fructose. This is of particular interest to diabetics.
On the one hand, D - fructose can be phosphorylated by a hexokinase , the first enzyme in glycolysis, just like glucose, and thus be introduced into glycolysis. In an alternative way, the fructose is converted by the enzyme fructose kinase to fructose-1-phosphate , which is further broken down by the fructose-1-phosphate aldolase to dihydroxyacetone phosphate . This in turn is used directly in glycolysis.
D - Galactose can be converted into glucose-6-phosphate via the intermediate stages galactose-1-phosphate and UDP-galactose , which flows into the glycolysis as usual.
In addition to single sugars, double sugars can also be processed, provided that enzymes are present that break them down into their components. Thus sucrose by invertase separated into its components glucose and fructose, which enter into the glycolysis as described. The same happens with lactose , which is split into galactose and glucose by the enzyme β-galactosidase . The same applies to polysaccharides . In order to use starch from grain, for example, the seeds are germinated. The plant's own enzyme amylase breaks down the starch into maltose , which in turn can be processed by the yeast.
Alternative way
The bacterium Zymomonas mobilis is also able to produce ethanol from glucose. It only uses part of the metabolic pathway described above. Instead of glycolysis, the glucose is broken down by the Entner-Doudoroff path to pyruvate and glyceraldehyde-3-phosphate . Glyceraldehyde-3-phosphate can be introduced into glycolysis and also broken down into pyruvate. The last two steps of alcoholic fermentation are the same as for yeast. Only one molecule of ATP can be obtained from one molecule of glucose. The fermentation runs faster in this way than that used by yeast and achieves a higher yield. Z. mobilis is used to make pulque from agave juice.
Fermentation by-products
Fermentation by-products or alcoholic accompanying substances are produced in addition to ethanol and carbon dioxide during alcoholic fermentation. Some of these by-products are known as fusel oils . They can also be detected when a pure glucose solution is fermented. When brewing beer, the difference in taste between wort and green beer or beer indicates that fermentation by-products have arisen. They contain, for example, higher alcohols such as n-propanol, isobutanol, 2-methylbutanol, 3-methylbutanol and aromatic alcohols such as 2-phenylethanol , tyrosol or tryptophol . In addition, contact esters such as ethyl acetate, phenyl acetate and i-amyl acetate on. Carbonyl compounds such as aldehydes , for example acetaldehyde, propanal, butanal or furfural, as well as ketones and diketones . Sulfur compounds such as H 2 S , SO 2 , ethyl mercaptan and methyl mercaptan occur in small amounts.
Further, also are organic acids such as acetic acid , lactic acid , pyruvic acid , 2-acetolactic acid and fatty acids (C 4 -C 12 ) formed. Polyhydric alcohols such as glycerine , 2,3-butanediol and 2,3-pentanediol also occur as fermentation by-products. The substances listed are the most important representatives of the individual groups.
Natural appearance
Microorganisms can be found everywhere in nature . Fruit is also covered with bacteria and yeasts that cannot be completely removed by simply washing. If fruit is left in a warm environment for a long time after harvest, these microorganisms multiply. They break down cell structures and penetrate the interior of the colonized fruit. This becomes visible, for example, as a soft spot or a brown spot on an apple. During this decomposition process, there may be a lack of oxygen in places, especially inside the fruit. The yeast cells living there switch their metabolism to alcoholic fermentation. In this way, it is possible that spoiled fruit can contain a significant amount of alcohol.
Until the middle of the 19th century it was completely unknown that the wild yeasts that naturally colonize the fruit multiply and form alcohol from the sugar in the liquid. Some winemakers still use it to this day that after the grapes are pressed into a mash, all the yeast strains that happen to settle on the skins should multiply in the liquid. This is known as spontaneous fermentation . Yeasts were not used in winemaking until the 20th century. Without the addition of cultured yeast, it takes a little longer because the concentration of natural yeast is very low at first. The result is largely left to chance; the different wild yeasts give the wine a more individual note. These yeast strains can also differ depending on the growing area of the grapes, which is why wines produced in this way can be assigned to a growing area in terms of taste. Common yeast species are Kloeckera apiculata and Saccharomyces exiguus . If winemakers rely on natural yeasts, they run the risk that undesirable yeasts and bacteria that live on the grape skin will multiply during the production process. If they get out of hand, the mash will spoil. For this reason, particularly suitable yeast strains have been bred for a long time in order to produce the desired wine aromas. These pure yeasts consist of only one yeast strain and are often specialized in one type of grape. Since freeze-drying can be used to preserve yeasts, such pure yeasts can easily be bought in large quantities, can be kept for many months and are easy to handle. If you add them in sufficient quantities at the beginning of the fermentation, the alcohol level rises quickly and the unwanted wild yeasts die off.
Use by humans
Pure yeasts
Today, pure yeast is mostly used for alcoholic fermentation . Depending on the fermentation conditions, a suitable strain of yeast is chosen to achieve the desired result. To protect heat-sensitive ingredients, fermentation can be carried out using cold yeast at low temperatures (15–20 ° C). Alcohol-tolerant yeasts are used for port wine and sherry so that these drinks have an alcohol content of up to 16%. At the end of fermentation, the final alcohol content is adjusted with neutral alcohol. “Turbo yeasts” are new to the market. They have an even higher alcohol tolerance and, under optimal conditions, create up to 20% alcohol content. Drinks with an even higher alcohol content get this through distillation (“ burning ”), through the addition of neutral alcohol, which in turn is made through distillation from fermented raw materials, or through freezing of water.
The sugar content of pure fruit juices is not sufficient for alcohol contents above around 15–16%; sugar must be added. Turbo yeasts are mostly used to produce relatively high alcohol contents during fermentation without any appreciable taste, which are further increased by subsequent distillation. Their use in the production of wine or fruit brandies is not possible, since according to the law no sugar may be added to the fruit juice for these products.
beverages
There are a variety of alcoholic beverages whose alcohol content is due to alcoholic fermentation. In any case, a sugar-containing raw material is required.
beer
In beer , the starting material is starch , mostly from grain, which is broken down into sugar in a first step (saccharification). The most common method of saccharification of the starch in beer brewing today is that enzymes are first activated in the brewing grain by malting , which then split the starch into maltose (malt sugar) during mashing . When preparing the wort, the sugar is dissolved. The cooled wort is mixed with yeast and caused to ferment. The maltose is broken down by the brewing yeast (e.g. S. cerevisiae , Saccharomyces uvarum = top-fermenting, Saccharomyces carlsbergensis = bottom-fermenting) into ethanol and carbon dioxide.
Wine

Sugar-containing grape juice is used as the raw material for the production of wine . The wine yeast can ferment with a sugar concentration of up to 250 g per liter, in addition the osmotic pressure is too high and the water is drawn from the yeast cells. In order to keep the water in the cells for as long as possible, the yeast produces compatible solutes , mainly glycerine . The yeast can only ferment sugar up to a certain alcohol content; at higher contents it dies. The exact limit depends on the yeast and is between 5% and 23%. The ethanol content protects the wine from mold and other undesirable microorganisms during fermentation . Towards the end, fermentation is often kept in a reductive state in the absence of air , so that acetaldehyde is hydrogenated into ethanol and carbon dioxide . The aim is an oxidation of the flavors of ethanol and to the further oxidation of acetic acid by bacteria can be prevented. Carbon dioxide escapes in the process. Seasonally fermented grape must is offered in stores as New Wine ("Federweißer").
Sparkling wine
In the production of sparkling wine , a base wine with residual sugar content from higher-fermenting yeast strains ( Saccharomyces bayanus ) , which are also known as post-fermentation, sparkling wine or champagne yeast, is further fermented in a pressure vessel (e.g. in a bottle). This prevents the carbon dioxide produced from escaping.
Bakery products
One of the most important areas of application for fermentation is the production of baked goods. Baker's yeast ( Saccharomyces cerevisiae ) is used in the production of almost all types of bread and rolls as well as traditional cakes with yeast dough to loosen the dough . While the dough "rises", alcoholic fermentation creates the gas carbon dioxide. This is distributed finely in the dough and can increase its volume considerably. The resulting ethanol evaporates during the subsequent baking process, at the beginning of which the yeast dies due to the high temperatures. Toasted bread can still contain up to 2.8% alcohol by volume if alcohol has been added as a preservative after the baking process.
More food
An alcoholic food is from milk obtained kefir . It is made from kefir grains, a mixture of different types of symbiotic yeast and bacteria. The lactose contained in milk is broken down by the bacteria via lactic acid fermentation to lactic acid and by the yeasts via alcoholic fermentation to form ethanol. The alcohol content can be 0.2 to about 2% depending on the duration of fermentation. Asian steppe peoples traditionally drink kumys , fermented mare's milk .
Industry
Ethanol is also used as a vehicle fuel. It is also used in many technical processes, is the starting material for chemical syntheses or is used for disinfection . This ethanol is also produced by alcoholic fermentation of yeast. Basic materials are cheap grain or potatoes, the starch of which is broken down into sugar by industrially produced enzymes. Here too, fermentation cannot exceed a maximum alcohol content of 23%. The subsequent column distillation gives a content of 96% ( azeotrope ). Since alcohol produced in this way would be enjoyable, it is also subject to spirits tax in Germany . The ethanol used for fuel production, which is subject to the strictest official controls, is an exception. Also, no spirits tax has to be paid on denatured ethanol. A small amount of methyl ethyl ketone and other substances are added for denaturation . This makes it inedible and this excludes consumption in food and beverages.
literature
- Gerolf Annemüller , Hans-Jürgen Manger, Peter Lietz : The yeast in the brewery. Yeast management - culture yeast - yeast cultivation - yeast propagation in the beer production process. Experimental and educational institute for brewing (VLB), Berlin 2005, ISBN 3-921690-50-1 .
- Ernst E. Bruchmann: Applied biochemistry. Food chemistry, fermentation chemistry, agrochemistry. Ulmer, Stuttgart 1976, ISBN 3-8001-2301-0 (somewhat out of date).
- Helmut Hans Dittrich, Manfred Großmann: microbiology of wine. 3. Edition. Ulmer, Stuttgart 2005, ISBN 3-8001-4470-0 .
- Adam Maurizio: History of Fermented Drinks. Reprint. Sandy, 1993, ISBN 3-253-02199-8 .
- Lubert Stryer : Biochemistry. 6th edition. Spectrum, Heidelberg 2007, ISBN 978-3-8274-1800-5 .
Web links
Individual evidence
- ^ E. Racker: History of the Pasteur effect and its pathobiology. In: Mol Cell Biochem. 5 (1-2), 1974, pp. 17-23. PMID 4279327 . doi: 10.1007 / BF01874168
- ↑ Eduard Buchner: Alcoholic fermentation without yeast cells. In: Reports of the German Chemical Society. 30, 1897, pp. 1110-1113, doi: 10.1002 / cber.189703001215 .
- ↑ E. Negelein, HJ Wulff: Diphosphopyridinproteid, alcohol, acetaldehyde. In: Biochemical Journal . Springer, Berlin 293, 1937, pp. 352-389.
- ^ Klaus Koschel: The development and differentiation of the subject chemistry at the University of Würzburg. In: Peter Baumgart (Ed.): Four hundred years of the University of Würzburg. A commemorative publication. (= Sources and contributions to the history of the University of Würzburg. Volume 6). Degener & Co. (Gerhard Gessner), Neustadt an der Aisch 1982, ISBN 3-7686-9062-8 , pp. 703-749; here: p. 729.
- ^ W. Furey et al .: Structure-function relationships and flexible tetramer assembly in pyruvate decarboxylase revealed by analysis of crystal structures. In: Biochimica et Biophysica Acta (BBA). Springer, Berlin 1385, 2, 1998, pp. 253-270. PMID 9655915 .
- ↑ H. Eklund et al .: Crystallographic investigations of alcohol dehydrogenases. In: EXS. Birkhäuser, Berlin 71, 1994, pp. 269-277. PMID 8032158 .
- ↑ PC Hinkle: P / O ratios of mitochondrial oxidative phosphorylation. In: Biochimica et Biophysica Acta (BBA). Springer, Berlin 706, 1-2, 2005, pp. 1-11. PMID 15620362 .
- ↑ JP van Dijken, RA Weusthuis, JT Pronk: Kinetics of growth and sugar consumption in yeasts. In: Antonie Van Leeuwenhoek. In: International journal of general and molecular microbiology. Springer, Dordrecht 63, 3-4, 1993, pp. 343-352. PMID 8279829 .
- ↑ K. Tonomura: Ethanol fermentation in bacteria. In: Seikagaku. The journal of the Japanese Biochemical Society. Gakkai, Tokyo 59,10,1987, pp. 1148-1154. ISSN 0037-1017
- ↑ TW Kimmerer, RC McDonald: Acetaldehyde and Ethanol Biosynthesis in Leaves of Plants. In: Plant Physiology. Rockville Md 84, 4, 1987, pp. 1204-1209, PMC 1056752 (free full text, PDF).
- ↑ Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-144861-3 , pp. 378-379.
- ↑ E. Oberdisse, E. Hackenthal, K. Kuschinsky: Pharmacology and Toxicology . Springer, 2002, Berlin, Heidelberg, New York, ISBN 978-3-642-62634-0 , p. 791.
- ↑ J. Piskur and others: How did Saccharomyces evolve to become a good brewer? In: Trends in Genetics . Elsevier, Amsterdam 22, 4, 2006, pp. 183-186. PMID 16499989 .
- ↑ J. Blom, MJ De Mattos, LA Grivell: Redirection of the Respiro-Fermentative Flux Distribution in Saccharomyces cerevisiae by Overexpression of the Transcription Factor Hap4p. In: Applied and Environmental Microbiology . Washington DC 66, 5, 2000, pp. 1970–1973, full text as pdf .
- ^ T. Conway: The Entner-Doudoroff pathway, history, physiology and molecular biology. In: Federation of European Microbiological Societies. Blackwell, Oxford 9, 1, 1992, pp. 1-27. PMID 1389313 .
- ^ Food lexicon, edited by Waldemar Ternes. books.google.de, accessed on November 19, 2009 .
- ↑ mondovino.ch: Increasing alcohol content in wine
- ↑ weinimwww.de: How high should the alcohol content of a wine be - and what does it say?
- ↑ Helmut Hans Dittrich, Manfred Großmann: Microbiology of Wine. 3. Edition. Verlag Eugen Ulmer, Stuttgart 2005, p. 39.
- ↑ Helmut Hans Dittrich, Manfred Großmann: Microbiology of Wine. 3. Edition. Verlag Eugen Ulmer, Stuttgart 2005, pp. 22 and 42
- ↑ focus.de
- ↑ Investigation of the Cantonal Laboratory of the Health Directorate in Zurich cleankids.de